Understanding Parkinson’s Disease Progression Through Protein Biomarkers
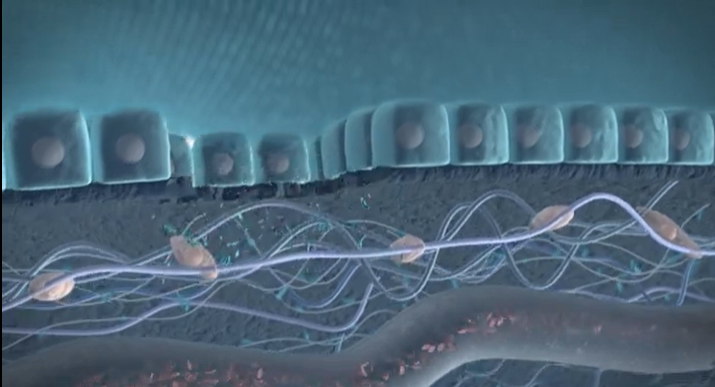
Parkinson’s Disease (PD) is a complex neurodegenerative disorder, primarily affecting the brain’s control over movement, thought, memory, and emotion. Early symptoms often manifest as tremors due to impaired motor skills. Underlying these visible symptoms is a cascade of molecular changes, beginning with alterations in specific proteins—one of which, α-synuclein, plays a critical role in PD pathology. In Figure 1, we have illustrated how α-Synuclein aggregates, which impairs the motor neuron.
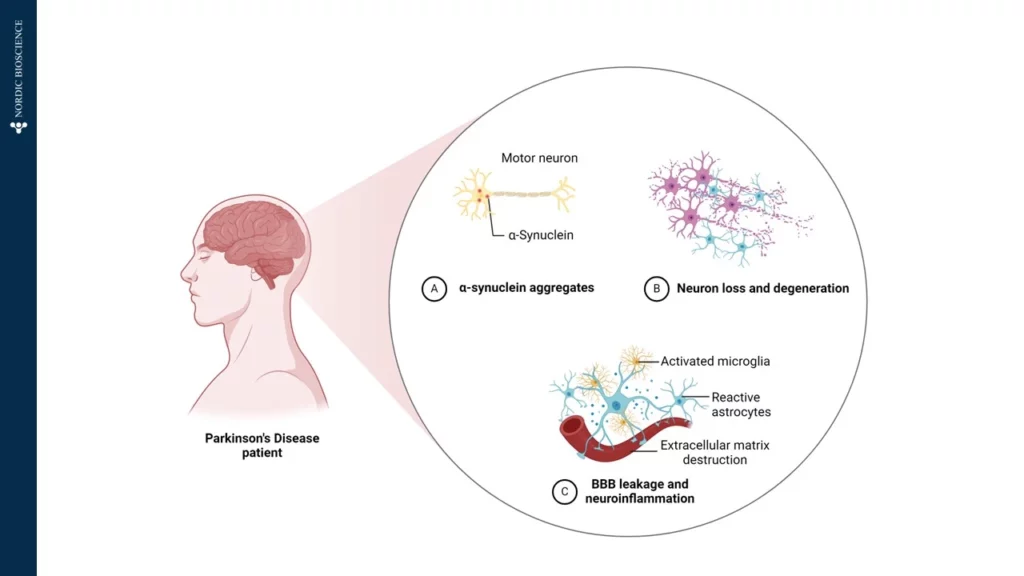
In a healthy brain, α-synuclein supports neuronal communication. However, in Parkinson’s, this protein undergoes abnormal processing, driven partly by the enzyme Calpain-1, which cleaves α-synuclein into smaller, altered fragments. This early cleavage disrupts cellular function and promotes the formation of toxic aggregates, which accumulate, kill neurons, and drive disease progression. Intriguingly, these fragmented proteins can cross the blood-brain barrier and enter the bloodstream, providing a potential “window into the brain” for tracking disease activity from a simple blood sample.
At Nordic Bioscience, we have developed an innovative approach to harness this biomarker potential. Using our ProteinFingerPrint Biomarker Technology™, we can detect Calpain-1-cleaved α-synuclein fragments in blood serum with high precision. Our specific assay, α-SYN-C, captures the unique “fragment fingerprint” of PD by quantifying these cleaved fragments, which are significantly elevated in the blood of PD patients compared to healthy individuals, as illustrated in Figure 2.
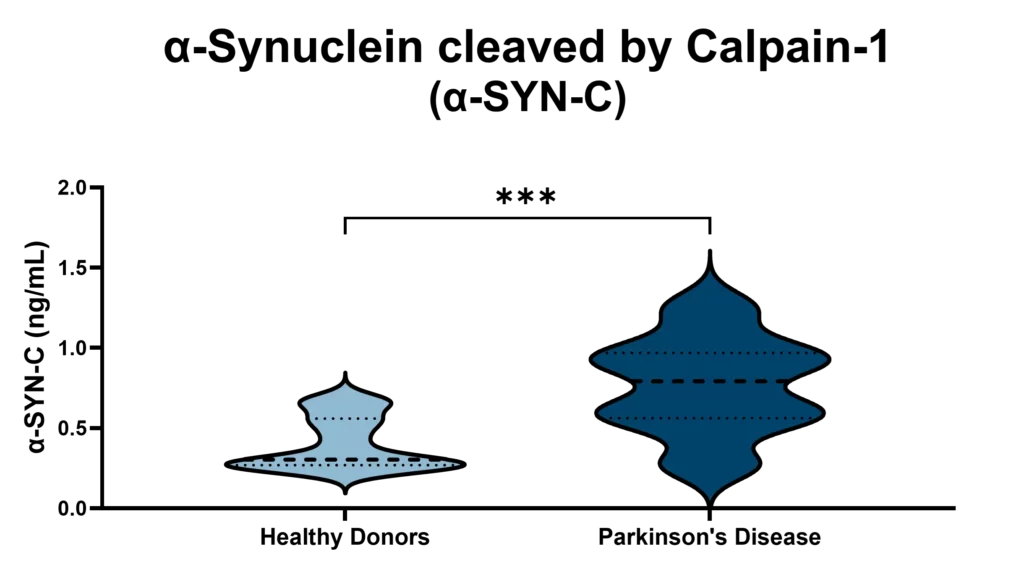
This biomarker offers a non-invasive, accessible tool for monitoring Parkinson’s Disease progression and evaluating therapeutic responses. By examining α-SYN-C levels in blood samples, our technology not only provides insights into PD mechanisms but also opens doors for developing targeted therapies that address the disease’s underlying pathology. Through this work, we aim to support more accurate PD diagnostics and more effective, individualized treatments in the fight against neurodegeneration.

 The
The