Fibroblast activity kills – Circulating endotrophin (PRO-C6) is prognostic for liver-related events in patients with cirrhosis from chronic hepatitis C
Introduction
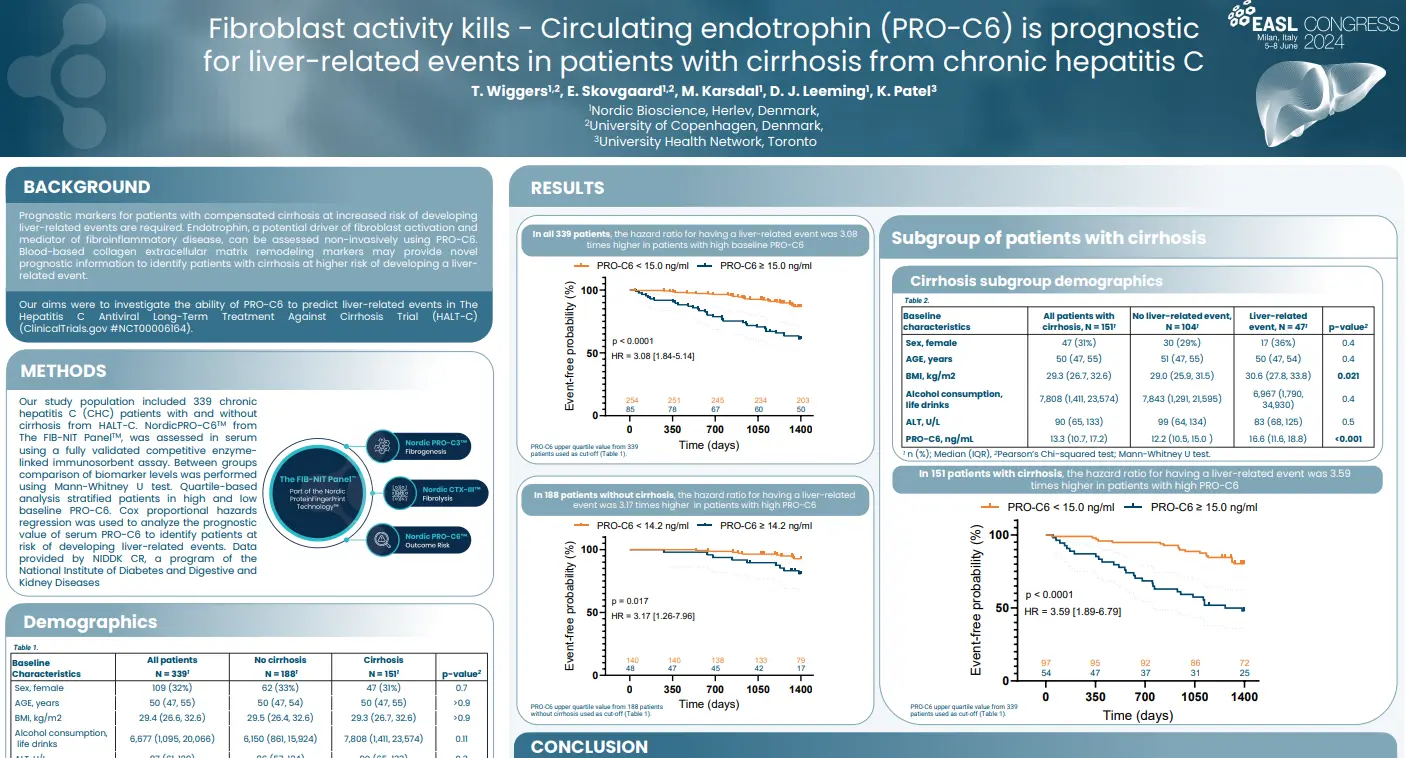
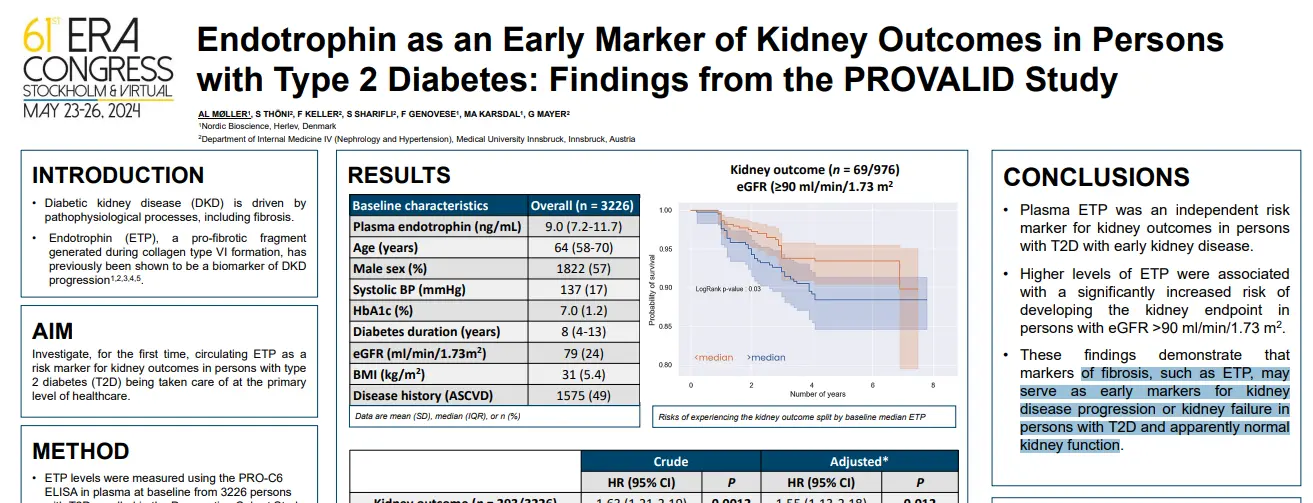
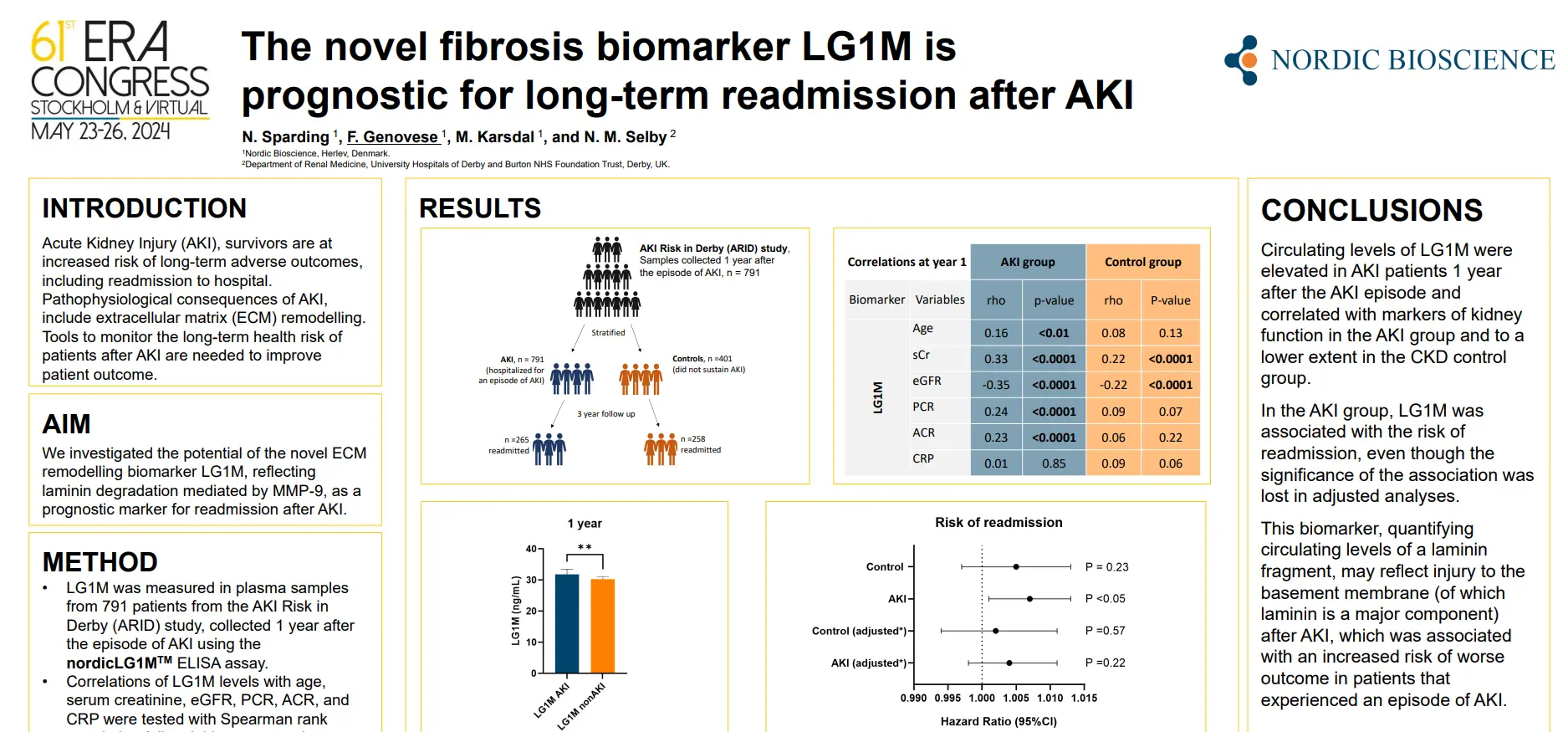
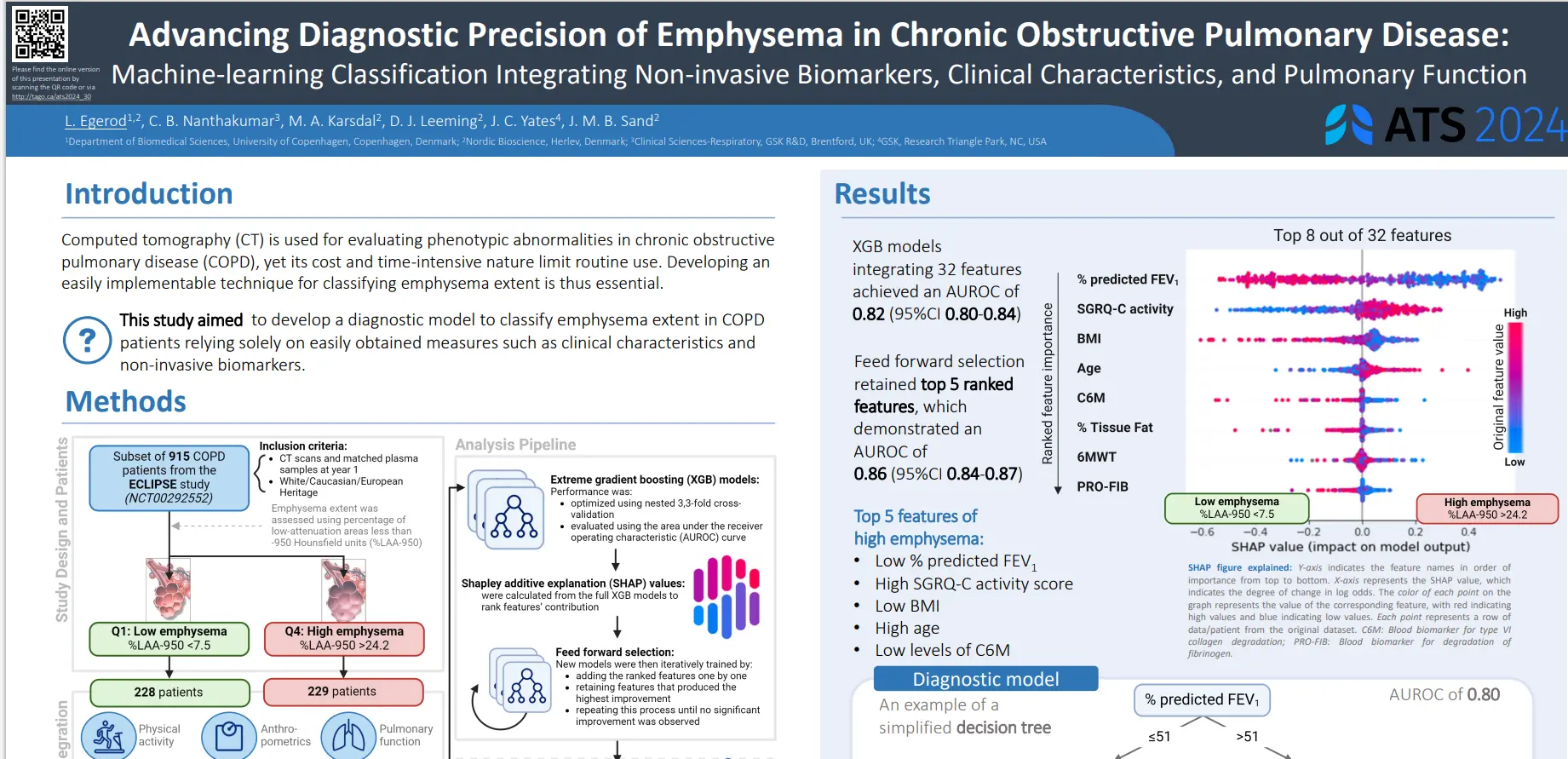
Prognostic markers for patients with compensated cirrhosis at increased risk of developing liver-related events are required. Endotrophin, a potential driver of fibroblast activation and mediator of fibroinflammatory disease, may be assessed non-invasively using nordicPRO-C6™. Blood-based collagen extracellular matrix remodeling markers may provide novel prognostic information to identify patients with cirrhosis at higher risk of developing a liver-related event.
In this study we aimed to investigate the ability of nordicPRO-C6™ to predict liver-related events in The Hepatitis C Antiviral Long-Term Treatment Against Cirrhosis Trial (HALT-C) (ClinicalTrials.gov #NCT00006164).
Poster
Conclusion
NordicPRO-C6™, a biomarker of circulating endotrophin, was primarily associated with an increased risk of developing a liver-related event in patients with cirrhosis from CHC. NordicPRO-C6™ is a potential monitoring blood-based marker that may also provide prognostic information in treatment-naïve CHC patients with moderate-advanced fibrosis at higher risk of developing a liver-related event. The prognostic utility of nordicPRO-C6™ in CHC patients after sustained virologic response and other advanced stage chronic liver disease is required.