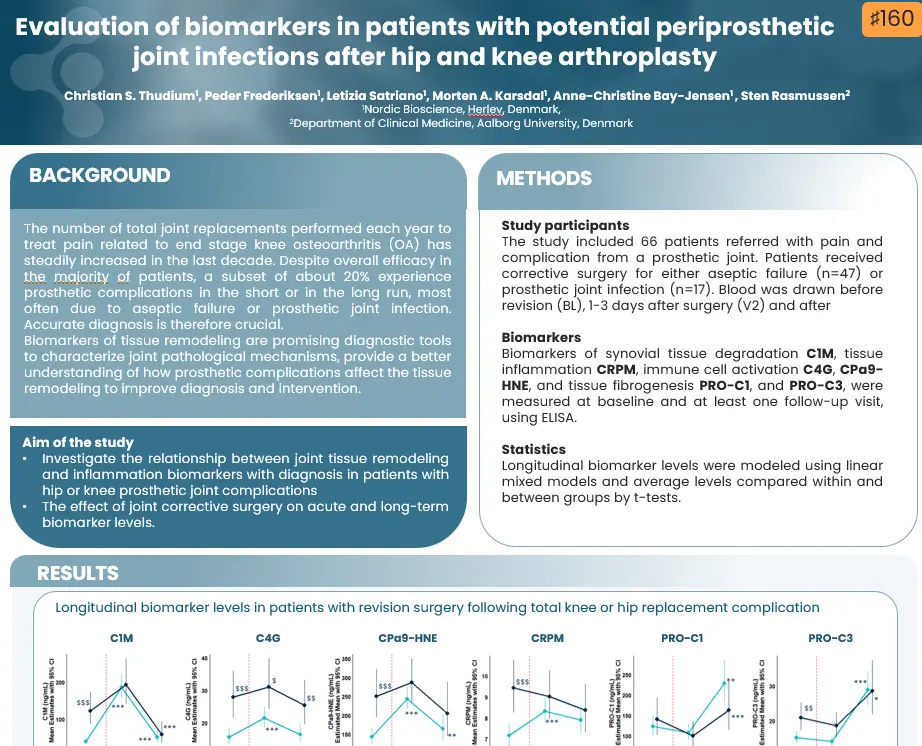
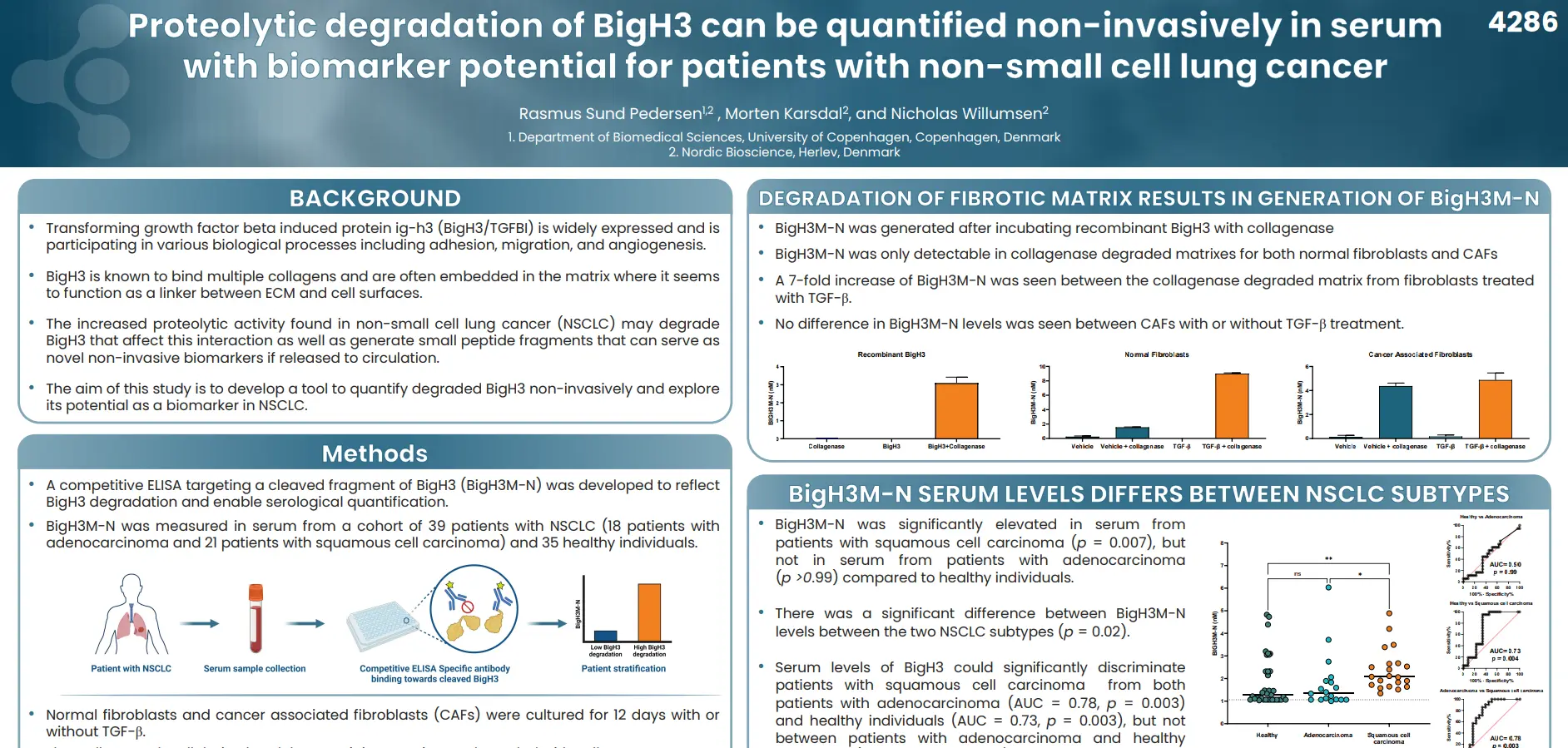
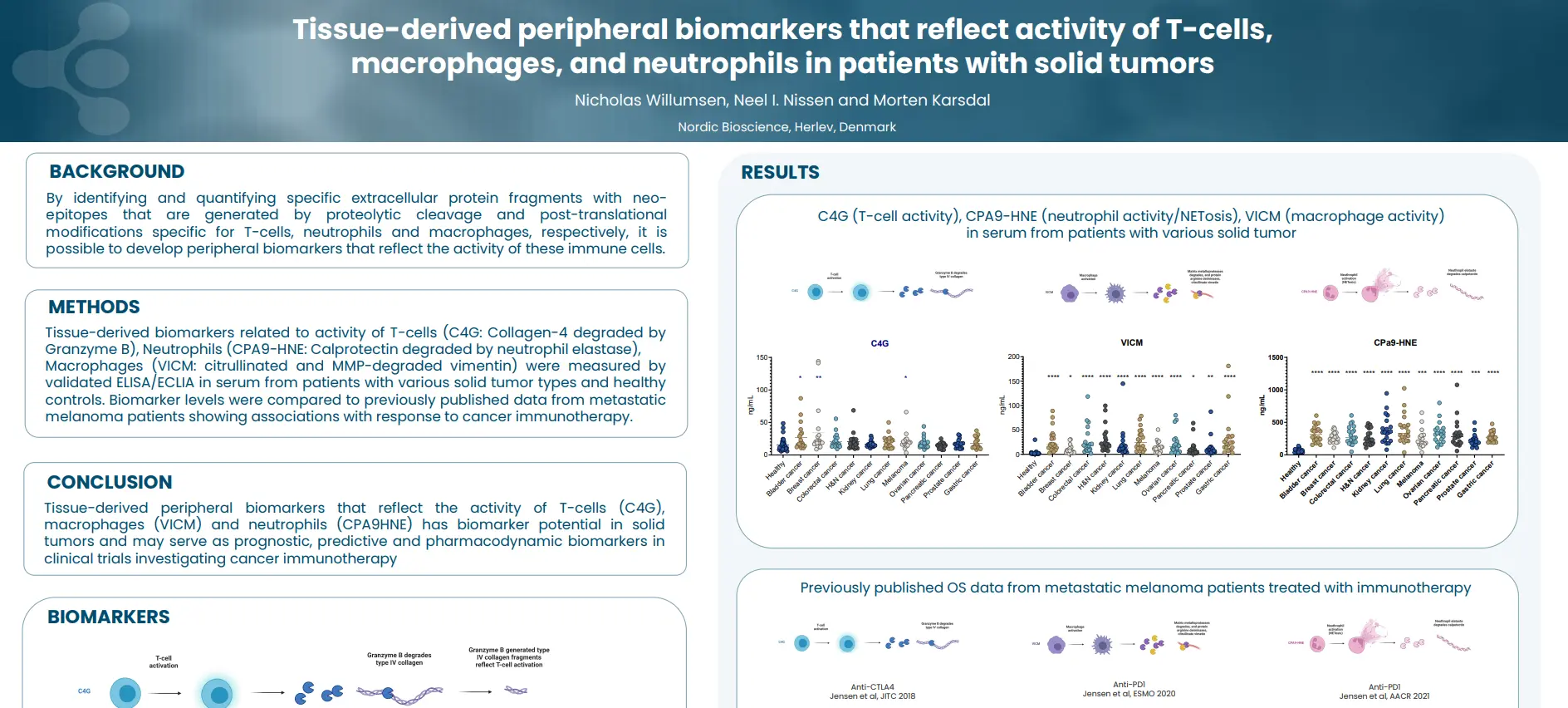
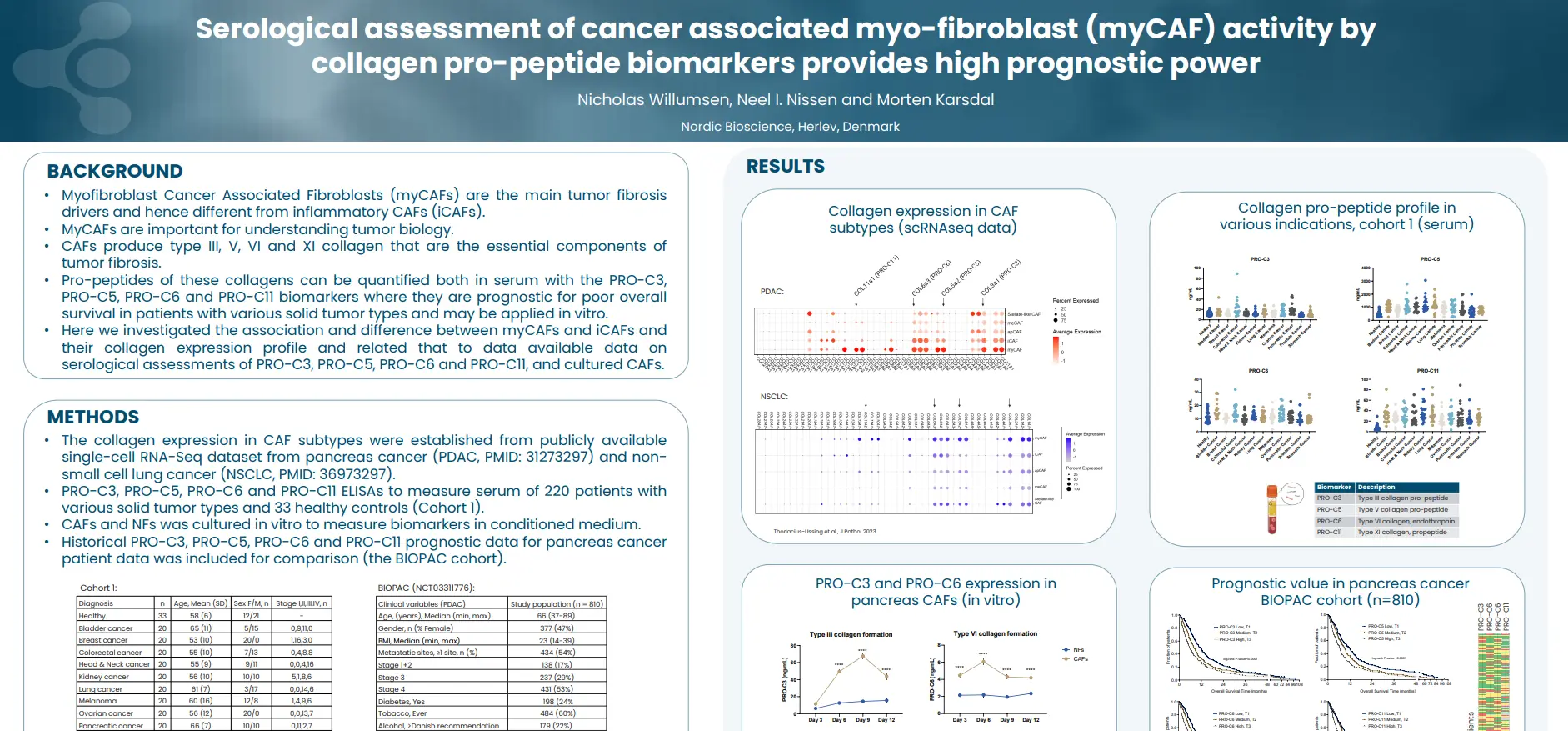
Regulatory enablement of molecular endotypes for drug development. Definition of the context of use (COU) and molecular endotype which most urgently will assist in drug development: Stakeholder alignment under the Clinical Trial Symposium (CTS) & OARSI umbrella
Introduction
For a biomarker to be considered in therapeutic development, it must navigate various approval pathways. In the US, acceptance for single therapeutic trials is via IND, NDA, and BLA submissions, while for multiple drug development programs, it’s through the Biomarker Qualification Program. This program specifically evaluates the biomarker itself, not the measurement method.
Alternatively, in the US, a biomarker test can be approved by CDRH, leading to a legally marketable In-Vitro Diagnostic (IVD). The De Novo classification offers a pathway for novel medical devices, based on risk classification. Additionally, the 510(k) submission demonstrates equivalence to predicate devices, while PMA evaluates Class III device safety and efficacy. In the EU, approval is through CE marking, aligning with US FDA pathways.
A biomarker for general drug development can be qualified by CDER through the Biomarker Qualification Program, resulting in a tool usable under specific COUs. This process involves collaborative efforts with regulatory agencies and stakeholders, often in consortia, to streamline qualification. Monitoring biomarkers play a pivotal role in medical product development, furnishing tangible evidence of treatment impact, while predictive biomarkers serve to mitigate trial risks and minimize failures by pinpointing responsive patient subgroups.
Poster
Conclusion
Defining the Context of Use (COU) within real-world or clinical study populations is essential for biomarker development. Following CLSI guidelines ensures technical robustness, while careful risk-benefit evaluation supports clinical relevance. The regulatory enablement of molecular endotypes is essential for drug development.