Alzheimer’s Disease, Multiple Sclerosis and Parkinson’s Disease are neurological disorders with devastating consequences for those affected and their families. These diseases are characterized by a lack of biomarkers specifically tracking disease progression and the effect of potential disease modifying therapies, leading to inadequate treatment possibilities and patient care.
In our recent webinar, “The Brain’s Breakdown: Biomarkers and ECM in Neuroinflammation and Neurodegeneration”, we invited world-renowned Alzheimer’s biomarker specialist Professor Charlotte Teunissen, Multiple Sclerosis treatment specialists Dr. Tobias Sejbæk and Nordic Bioscience’s Director of Neuroscience, Kim Henriksen, to share the current developments for biomarkers in neurologic diseases.
In Alzheimer’s Disease (AD), recently developed fluid biomarkers, such as phospho-Tau isoforms (pTau217), have shown the ability to predict disease progression and treatment response in both plasma and CSF (measured in our CAP/CLIA-certified lab). This clearly illustrates how far the neuro field has come and highlights the importance of such biomarkers or continuous drug development in AD.
For Multiple Sclerosis (MS) and Parkinson’s Disease (PD), the work focuses on identifying a panel of biomarkers reflecting different aspects of these complex diseases, including neuroinflammation, blood-brain barrier integrity and active neurodegeneration.
There are therapies and biomarkers available in MS, but we still lack refined biomarkers to monitor the ongoing efficacy of the drugs. Such biomarkers can be used to enrich patient populations with respect to responses, as well as supporting the development of new drugs. Biomarkers such as fragments of collagen type IV, which reflect turnover of the basement membrane of the blood-brain barrier, have shown promise.
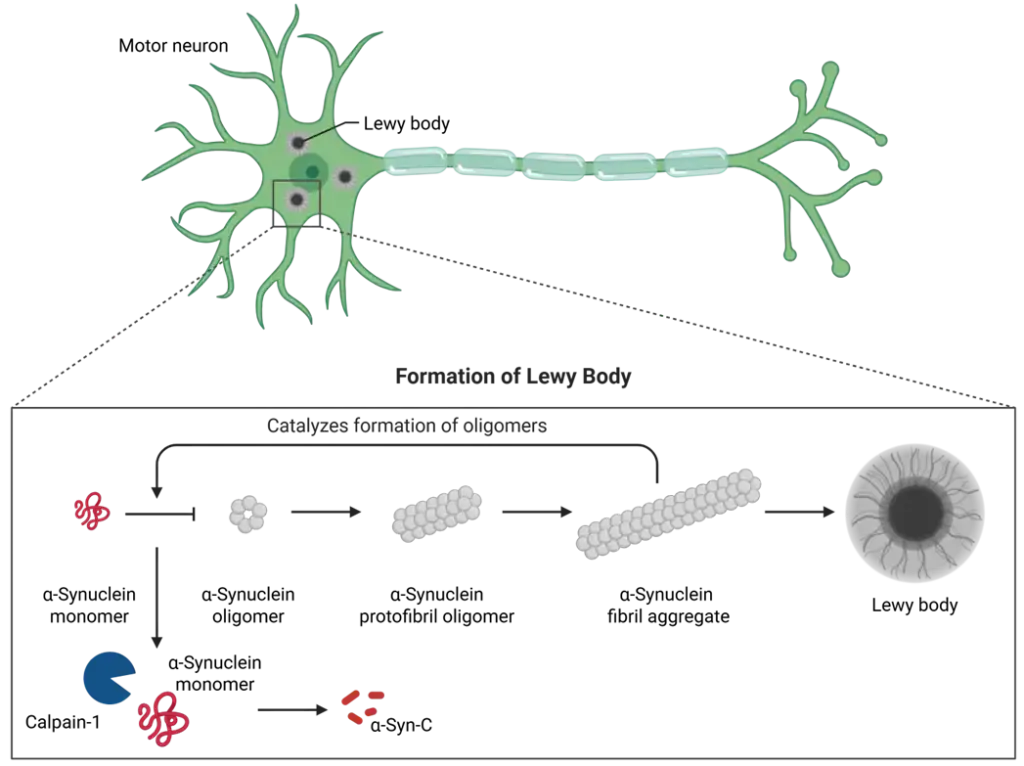
At the same time, in PD, there is a lack of biomarkers reflecting the underlying neuronal pathology especially in plasma/serum. Consequently, there is a competition on being the first to robustly detect the key protein α-synuclein—a biomarker that can capture the underlying pathology. Here, we introduced a novel assay detecting fragments of α-synuclein, which are generated during the loss of neurons. After further investigation, this biomarker could potentially become an important tool for drug development in PD.
Overall, fluid biomarkers for neurodegenerative diseases have come a long way in the last decade and are clearly critical to the success of drug development trials.


