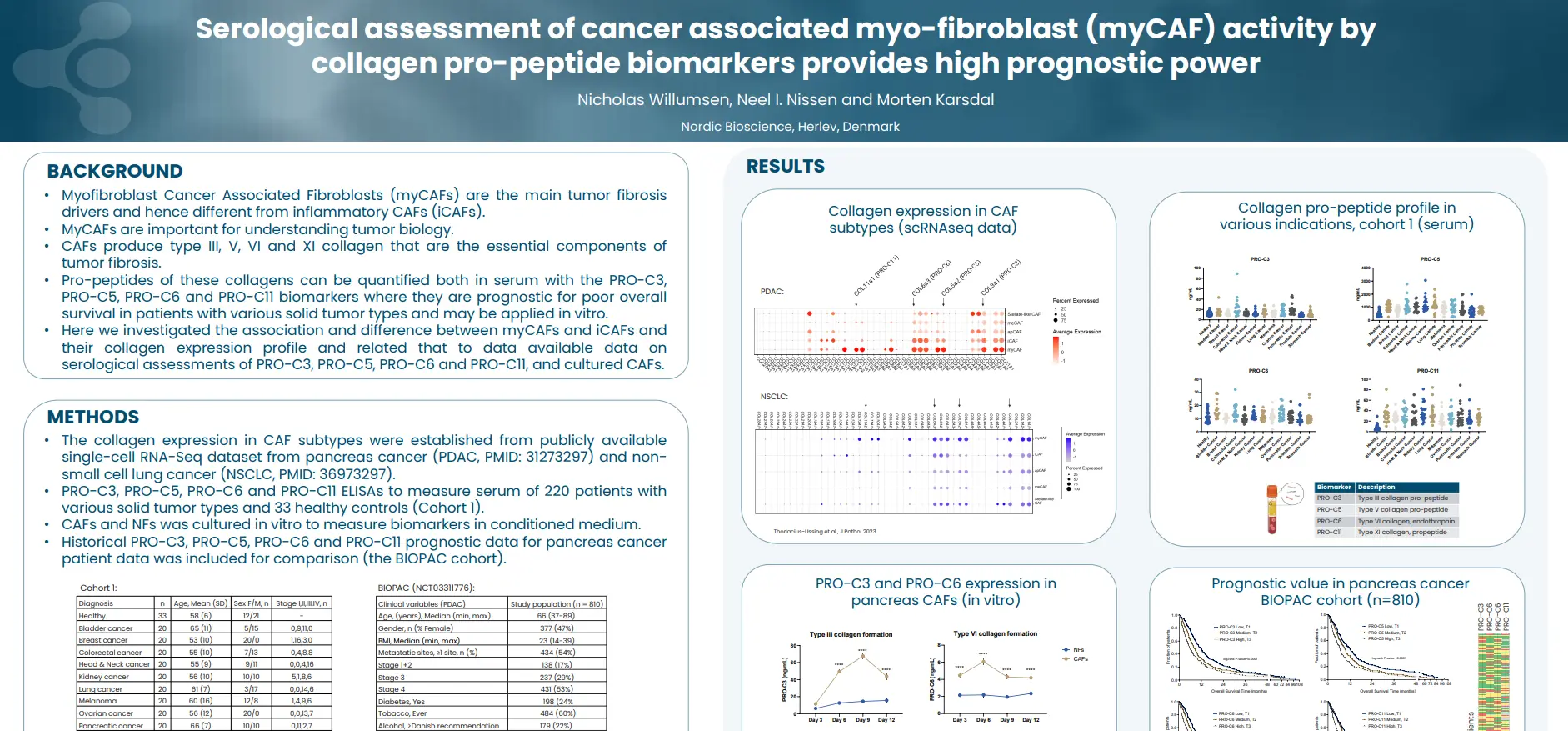
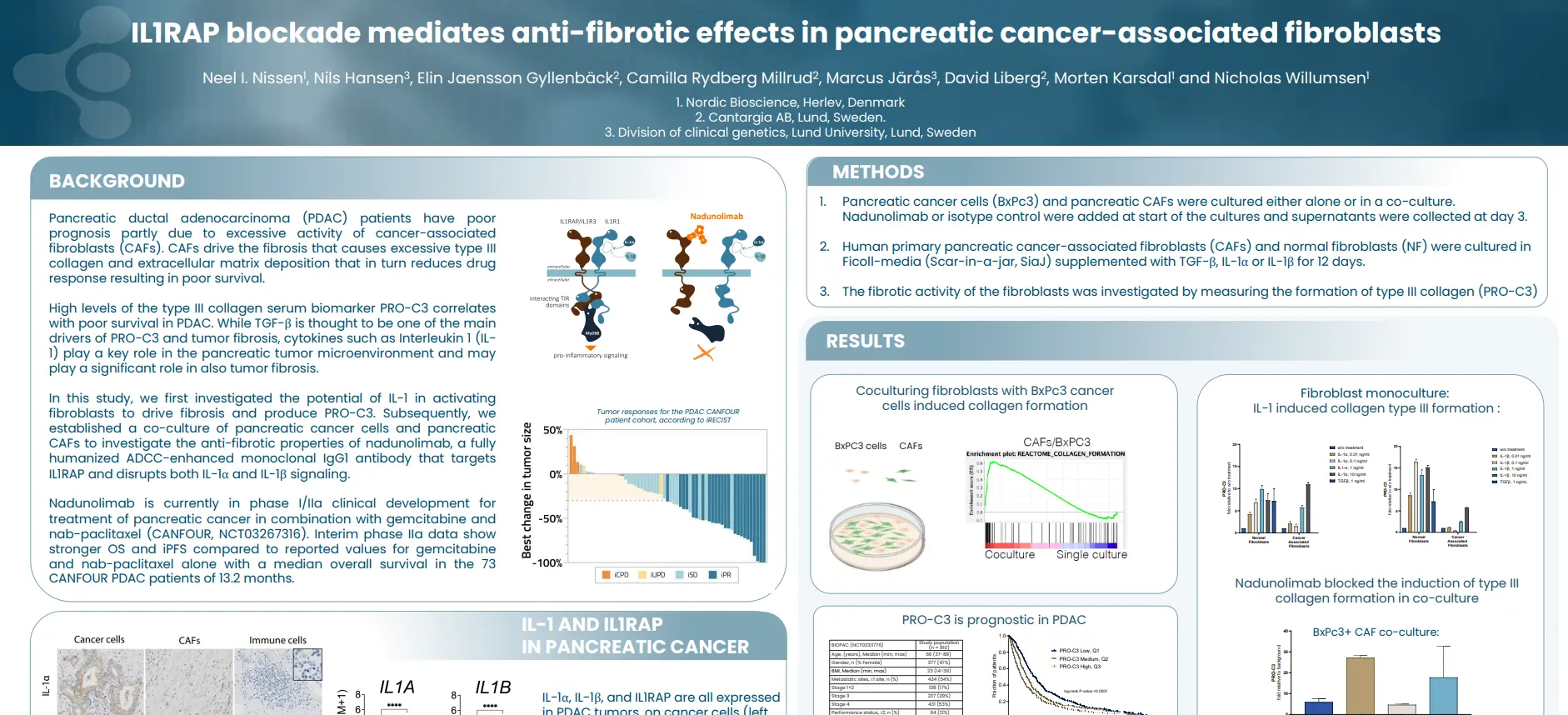
Join us for an insightful webinar, where experts will shed light on the multifaceted role of fibrosis in various health conditions.
This webinar offers a unique opportunity to gain comprehensive insights into the role of fibrosis in driving outcomes across various health conditions with a common component, providing valuable knowledge for researchers, clinicians, and healthcare professionals alike.
Agenda
- Fibroblast Activity Kills: The Basics of Extracellular Matrix in Health and Disease in Predicting Outcomes – Dr. Morten A. Karsdal
- The Extracellular Matrix of Cancers and The Modulation of the ECM – Dr. Raghu Kalluri
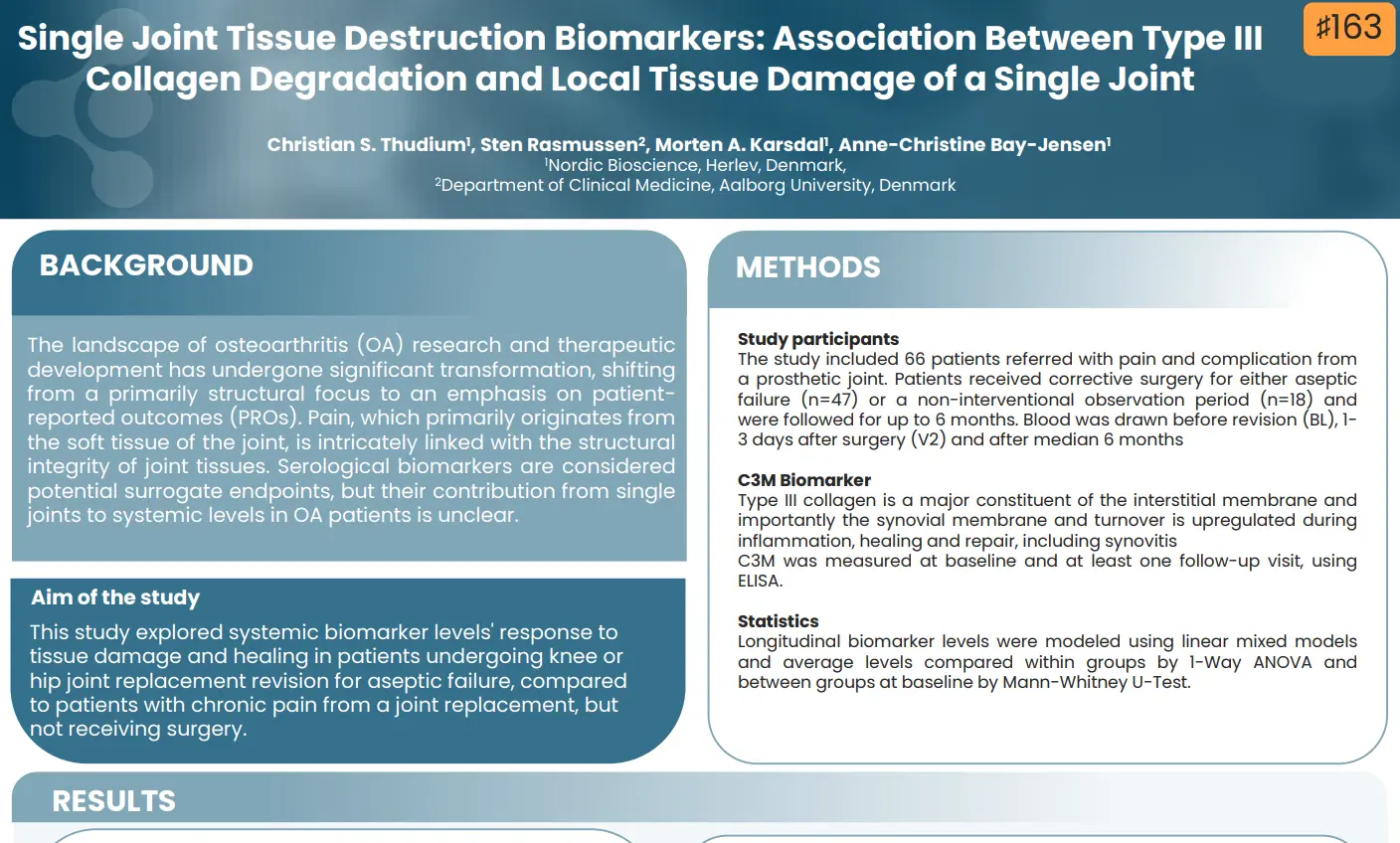
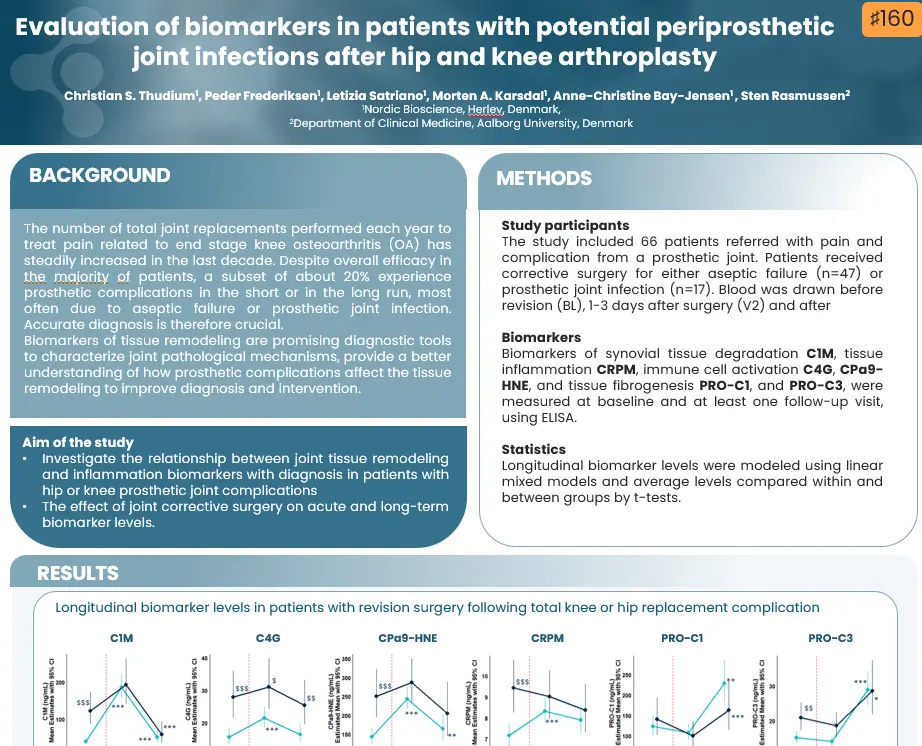
- Collagen biomarkers of Liver, Heart, and HCC Outcome – Dr. Diana Julie Leeming
- Q&A Break
- Liver Fibrosis and HCC – Cause or Consequence? – Dr. Scott Friedman
- Questions from the chat
Overview and speakers
Our presentations will cover a wide array of topics, starting with an exploration of the fundamentals of the extracellular matrix in health and disease, emphasizing the impact of fibroblast activity on outcomes. The complex relationship between liver fibrosis and hepatocellular carcinoma (HCC) will be explored, examining whether fibrosis is a cause or consequence of this condition.
Further discussions will delve into the extracellular matrix of cancers and the modulation of the ECM, providing insights into the interplay between fibrosis and cancer outcomes. Lastly, we’ll explore collagen biomarkers and their role in predicting outcomes related to liver, heart, and HCC.

Dr. Scott L. Friedman
- Dr. Scott L. Friedman is the Dean for Therapeutic Discovery and Chief of the Division of Liver Diseases at the Icahn School of Medicine at Mount Sinai. He is renowned for his pioneering research into the underlying causes of scarring, or fibrosis, associated with chronic liver disease, which affects millions worldwide.
- Dr. Friedman was among the first to isolate and characterize the hepatic stellate cell, the key cell type responsible for scar production in the liver, leading to significant advancements in the field.
- His groundbreaking work has led to the development of new anti-fibrotic therapies for liver disease that are currently reaching clinical trials, contributing to the translation of basic science into clinically meaningful advances. Dr. Friedman’s research has been continuously funded by the NIH since 1985, and he received his first faculty NIH grant (RO1) in 1986 at the age of 31.
- He has authored over 300 peer-reviewed publications and has been recognized with numerous awards, including the International Hans Popper Award in 2003 for his outstanding contributions to the understanding and treatment of liver disease.
- Dr. Friedman’s leadership as Chief of the Division of Liver Diseases at Mount Sinai has led to significant expansion, including increasing the faculty from 5 to 25 individuals and overseeing the creation of the largest liver fellowship in the United States.
- He is a fellow of multiple prestigious medical societies, including the American Gastroenterological Association, the American College of Physicians, and the American Association for the Study of Liver Diseases, among others.
Dr. Raghu Kalluri
- Dr. Kalluri is a medical researcher focused on the cellular microenvironment and exosomes in health and disease.
He has mentored over 200 research trainees and led numerous education and training initiatives at Harvard and MD Anderson Cancer Center. - He is co-Director of the MSTP, Assistant Dean of the graduate school, and Director of the Office of Mentoring and Training of Scientists at MD Anderson Cancer Center. His research has focused on extracellular matrix (ECM) biology, specifically basement membrane biology, and the discovery of several new ECM-derived endogenous angiogenesis inhibitors.
- He has contributed to our understanding of the role of TGF-β and BMP-induced epithelial to mesenchymal transition and mesenchymal to epithelial transition in organ fibrosis and cancer progression. His research has led to the identification of new therapeutic targets to combat organ fibrosis and the subsequent expansion of research on the cellular microenvironment to solid tumors, specifically pancreatic cancer.
- His research group has published over 350 papers in peer-reviewed journals, and his work has been translated into three successful Phase I clinical trials for cancer and the launch of five biotechnology companies.
Dr. Morten A. Karsdal
- Dr. Karsdal chairs the Extracellular Matrix Pharmacology Congress, an influential forum dedicated to advancing drug development by identifying changes in the ECM as common denominators in chronic diseases.
- Dr. Morten Karsdal joined Nordic Bioscience in 2001 and has been serving as CEO since June 2010.
- He is a prolific author, with more than 650 peer-reviewed articles to his name, accumulating over 34,000 citations and an h-index of 95.
- He is renowned for his expertise and commitment to biomarker development, extracellular matrix biology, and disease research, particularly in fibrosis, rheumatology, and diabetes. He possesses extensive experience in clinical trial design and the clinical application of biochemical markers, consulting for major pharmaceutical companies.
- As an honorary professor of inflammation research at the University of Southern Denmark, Dr. Karsdal supervises PhD students and contributes to advancing scientific knowledge in the field.
- In 2016, he authored the first edition of “Biochemistry of Collagens, Laminins and Elastin,” published by Elsevier Science, which is now in its 3rd edition, providing a comprehensive overview of structural proteins and their relevance in chronic diseases.
- Dr. Karsdal’s leadership and contributions to the Nordic Life Science community were recognized with the Arthur D. Little Nordic Life Science Award in 2023, highlighting his outstanding management and leadership achievements.
Dr. Diana Julie Leeming
- Dr. Diana Julie Leeming is the Senior Director of Fibrosis, Hepatic, and Pulmonary Research at Nordic Bioscience.
She joined Nordic Bioscience in 2004 and assumed the role of Director of Fibrosis in 2010, later being promoted to Senior Director in 2024. - Diana leads a team of 20 scientists and technicians dedicated to the development and clinical application of extracellular matrix (ECM) biomarkers in hepatic and pulmonary diseases with a fibrotic component. Her role involves overseeing the development of novel ECM biomarkers and implementing them in preclinical drug testing and clinical trials.
- Dr. Leeming focuses on developing serologically assessed markers to evaluate extracellular matrix remodeling in patients with pulmonary or hepatic fibrosis, aiding in diagnosis and pharmacodynamic evaluation.
- She is a principal inventor of the PRO-C3 assay, a fibrogenesis marker utilized in multiple clinical trial studies.
- Dr. Leeming has authored over 180 peer-reviewed publications, demonstrating her extensive contributions to the field. Her H-index is 61, her I10-index is 174, and her research has garnered over 11,825 citations as of March 2024.