700 Publications and 25+ Years of Research – Lessons for Today’s Clinical Studies
We are excited to invite you to a unique session in the ECM Pharmacology Symposium series, featuring a special presentation by Dr. Morten Karsdal, the Chair of the ECM Pharmacology Congress. This webinar will take us on a journey through 25 years of groundbreaking research and over 700 publications, highlighting how these insights can be applied in today’s clinical studies to improve patient outcomes.

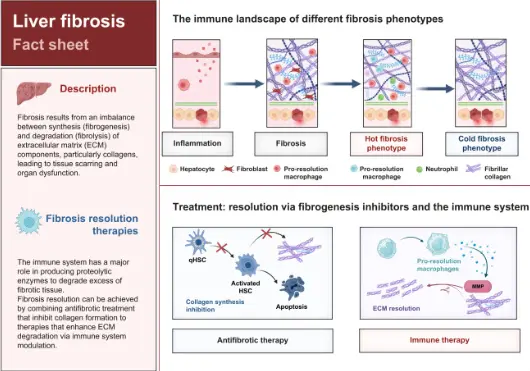
ECM remodeling is a fundamental driver of more than 50 chronic diseases, contributing to over 35% of deaths in the Western world. To restore organ function, we must understand and quantify ECM turnover—a crucial step in advancing patient care.
In this webinar, Dr. Karsdal will highlight the most influential findings from global collaborations and his own research, focusing on the balance between tissue formation and degradation in both healthy and diseased organs. With three letters of support from the FDA over the past three years emphasizing ECM biomarkers, this session will showcase real-world applications of ECM modeling in patient treatment.
Dr. Karsdal will share key findings from his own research and collaborations, highlighting how the structure of healthy organs changes when affected by disease. By examining the balance between tissue formation and degradation, we gain crucial insights into the progression of chronic diseases. The common mechanisms of ECM turnover in the lungs, liver, heart, kidneys, skin, joints, and intestines also apply to solid tumors, making ECM modulation a critical factor in treatment strategies. With FDA letters of support emphasizing ECM biomarkers and turnover, this session will showcase compelling real-world examples of how controlling ECM remodeling can directly benefit patients.
Scientific topics
We prepared nine key topics of the most prominent ECM research of the past 25+ years that you can apply in today’s clinical research.
These topics will explore how ECM remodeling influences disease progression, the role of biomarkers in precision medicine, and the impact of targeted interventions across multiple chronic conditions.

Key topics
1. The central role of ECM remodeling in over 50 diseases
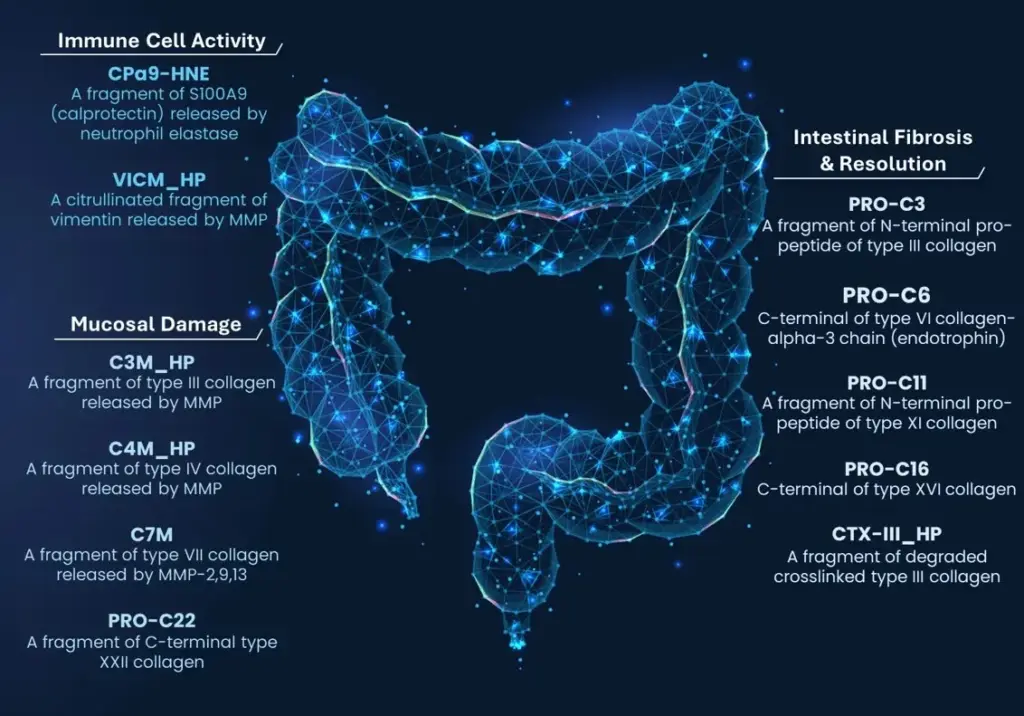
- Diagnostic, prognostic and pharmacodynamic biomarkers of pathological remodeling of the basement membrane and interstitial ECM
- Which pathologies are tissue formation or tissue destruction disorders?
- How may we use that to improve clinical study design and patient segregation – Key lessons learn from 25 years of biomarker research
2. Collagen formation outperforms liver biopsies in predicting outcome
- Collagen formation provides independent prognostic information in addition to the biopsies
- The difference between disease status and activity
3. Linking human genetic mutations to skin diseases
- Human genetic mutations linked to collagen type 6, 7 and 17 in systemic sclerocis (SSC), hidradenitis suppurutiva (HS), and atopic dermatitis (AD).
4. Doubling of response rates in solid tumors by serological assessment
- Doubling of response rates in solid tumors by serological assessment of type 3,5,8,11 and 12 collagens
- The myCAF collagens
5. The collagen hormone Endotrophin & the cardio-renal axis in the metabolic syndrome
- Understanding the organ death trajectory in obesity/metabolic syndrome of the heart, kidney and liver
- Endotrophin predicts outcome in CKD and HFpEF
- Understanding fibroblast activities as the central common denominator for outcomes
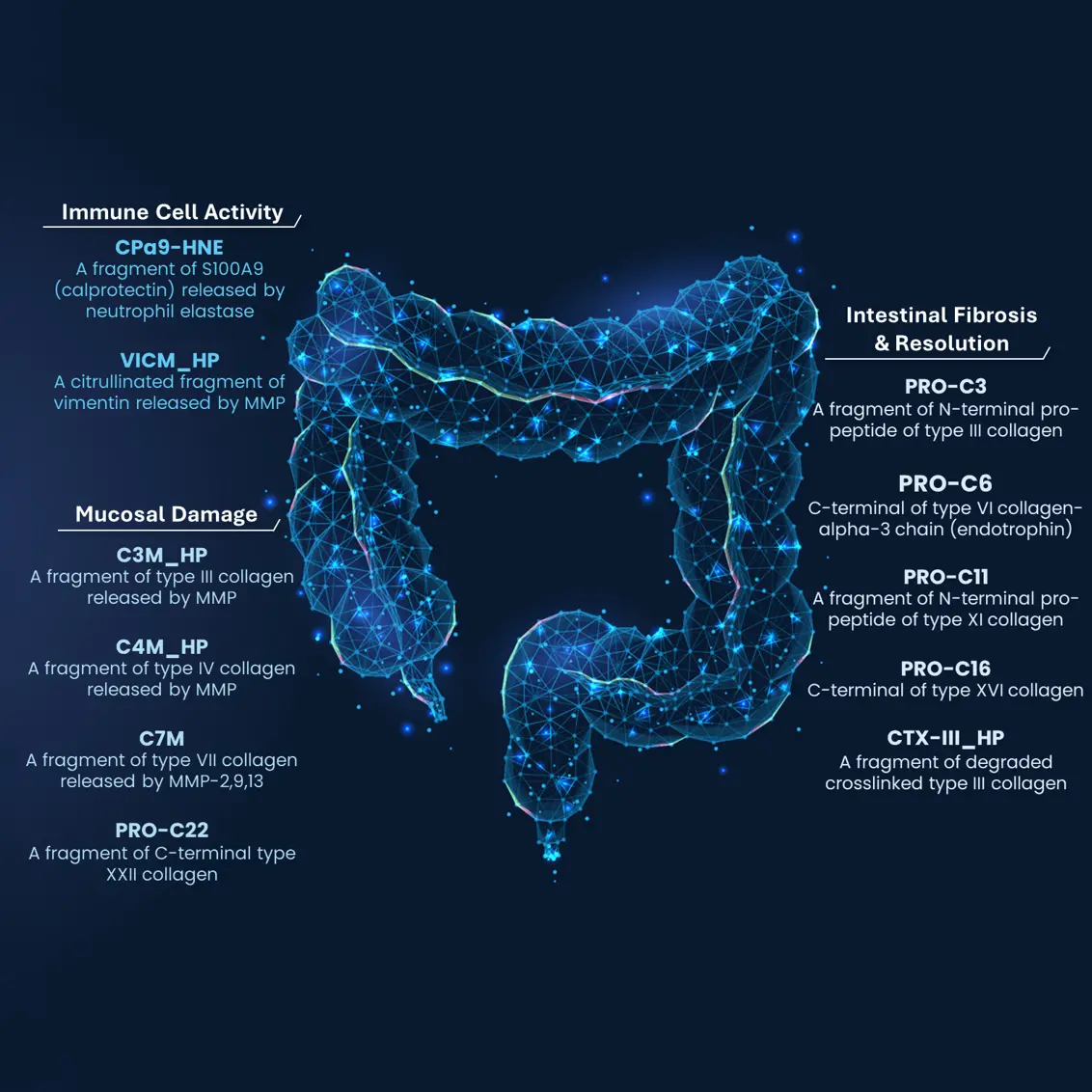
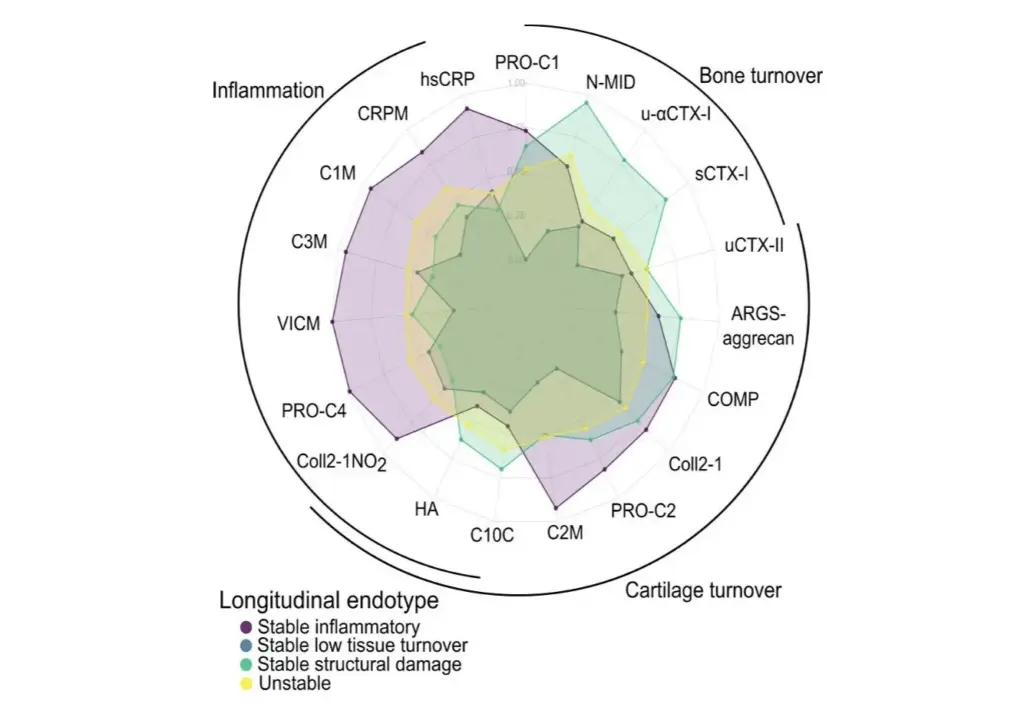
6. Endotyping fibrostenosis with genetically validated biomarkers
- Deconstructing IBD endotypes with human genetically validated biomarkers & biomarkers related to endoscopy scores and remission
7. Quantifying tissue destruction in auto-immune disorders
- We need to stop tissue destruction to be efficacious and reach ACR100 scores
8. Respiratory diseases in COPD and IPF
- Linking endotypes with disease trajectories that are treatable and actionable in drug development
- Elastin and collagen degradation/formation are prognostic for exacerbations, decline of lung function and death
9. Identification of treatable and druggable endotypes in osteoarthritis
- The difference between an illness and disease
- Providing better drug development paths for selected treatments.
Dr. Morten Karsdal
- Dr. Morten Karsdal joined Nordic Bioscience in 2001 and became CEO in June 2010, leading the company to significant advancements in biomarker development and disease biology.
- Dr. Karsdal is a KOL in extracellular matrix research, with more than 700 publication and an impressive H-factor of 100.
- Dr. Karsdal is an honorary professor of inflammation research at the University of Southern Denmark, where he continues to supervise PhD students, fostering the next generation of researchers.
- Dr. Karsdal chairs the Extracellular Matrix Pharmacology Congress, an important forum for advancing drug development by focusing on the extracellular matrix (ECM) as a key factor in most chronic diseases. He is renowned for his deep expertise in fibrosis, rheumatology (including rheumatoid arthritis and osteoarthritis), diabetes, and other chronic conditions, particularly in relation to ECM and biomarker research.
- Dr. Karsdal has led the development of FDA-approved and supported molecular diagnostics, as well as more than 100 commercialized biomarker assays, including ELISA assays and high precision automated platforms.
- He has extensive experience in clinical trial design and the clinical application of biochemical markers, often serving as a consultant to major pharmaceutical companies for the use of serological biomarkers in clinical trials.
- In 2016, he and his research team authored the first edition of “Biochemistry of Collagens, Laminins and Elastin,” published by Elsevier Science. The book, now in its 3rd edition as of 2023, is a key resource on collagens and structural proteins, with a focus on their applications in chronic diseases.
We are also excited to inform you about our upcoming in-person event, The Extracellular Matrix Pharmacology Congress, taking place in Copenhagen in June 2026. This congress will be a unique opportunity to gather with leading experts in the field and explore the latest advancements in extracellular matrix research and pharmacology.
Please stay tuned for updates as we prepare to bring you another engaging and educational webinar experience. Thank you for your patience, and we look forward to connecting with you soon!