Fibroblast derived type III collagen pro-peptides (PRO-C3) in plasma are associated with outcome for patients with NSCLC treated with anti-PD1 plus chemotherapy
Introduction
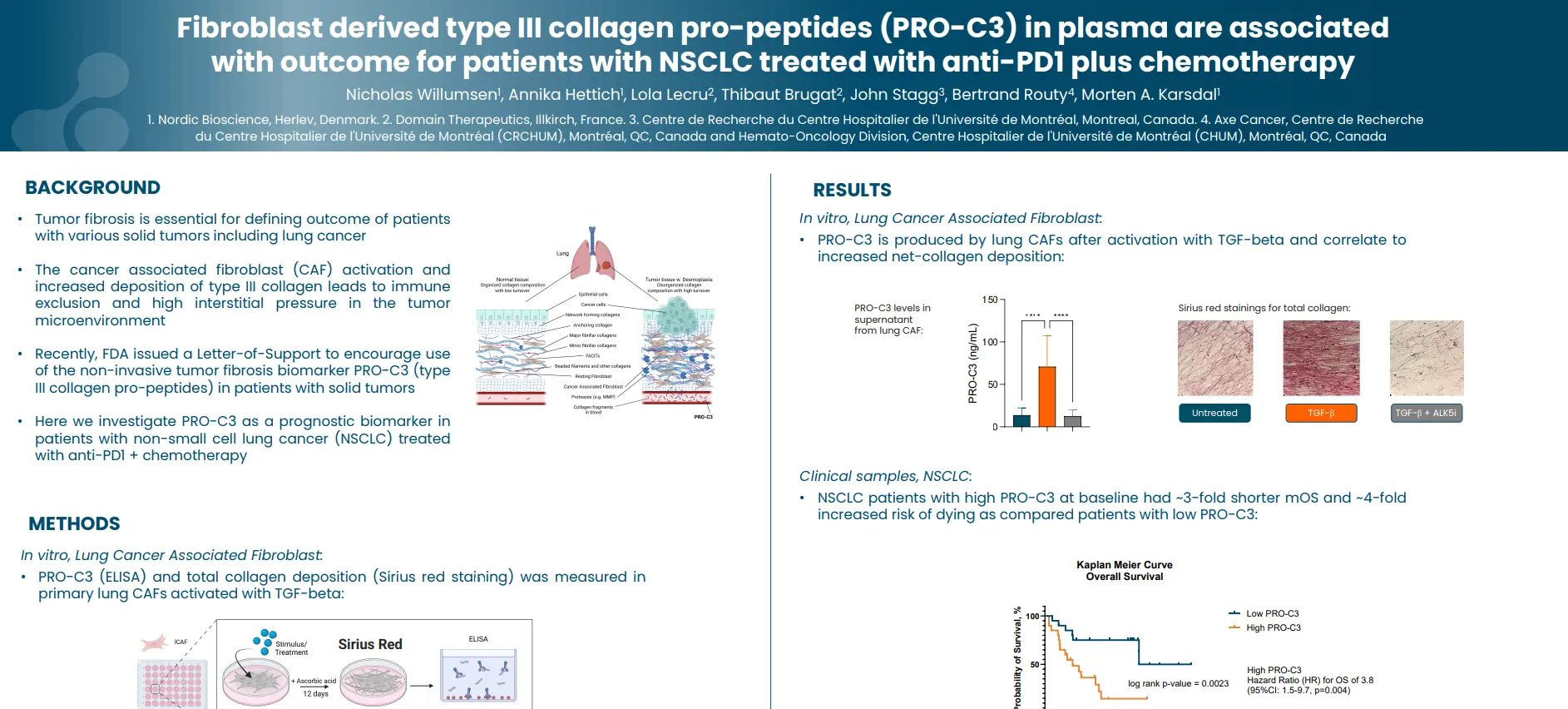
Tumor fibrosis is essential for defining outcome of patients with various solid tumors including lung cancer. The cancer associated fibroblast (CAF) activation and increased deposition of type III collagen leads to immune exclusion and high interstitial pressure in the tumor microenvironment. Recently, FDA issued a Letter-of-Support to encourage use of the non-invasive tumor fibrosis biomarker nordicPRO-C3™ (type III collagen pro-peptides) in patients with solid tumors.
In thi study we investigate nordicPRO-C3™ as a prognostic biomarker in patients with non-small cell lung cancer (NSCLC) treated with anti-PD1 + chemotherapy.
Poster
Conclusion
Type III collagen pro-peptides (nordicPRO-C3™) is produced by activated lung CAFs and is a surrogate of fibrogenesis. High nordicPRO-C3™ levels in pre-treatment plasma associate with poor outcome for patients with NSCLC treated with anti-PD1 plus chemotherapy. These findings suggest that quantifying tumor fibrosis is important for prognostication of patients with lung cancer.