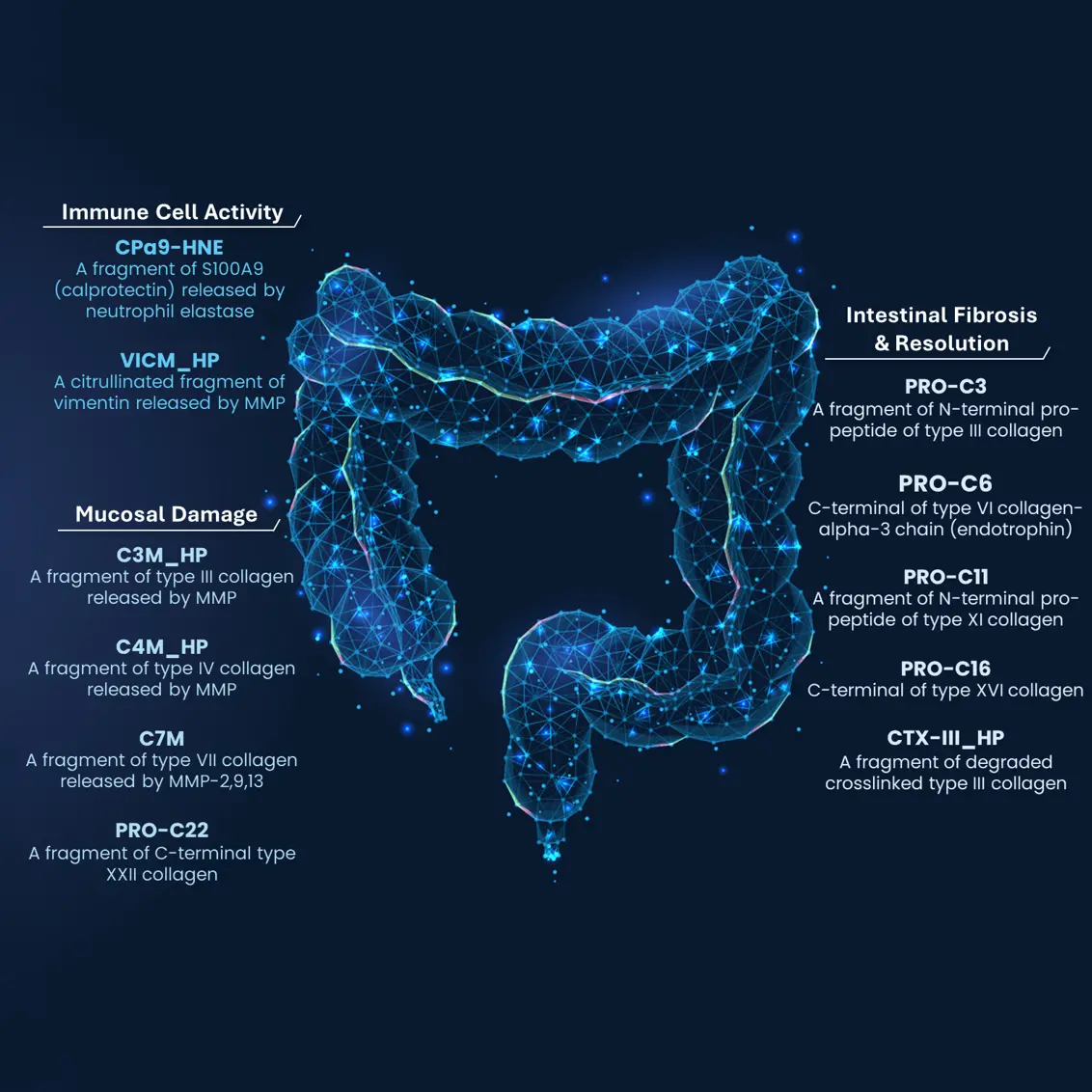
Assessing disease burden and treatment response in ulcerative colitis (UC) remains challenging, often leading to treatment withdrawal and unresolved disease activity. These challenges highlight the need for better, non-invasive biomarkers to monitor disease activity and predict treatment outcomes.
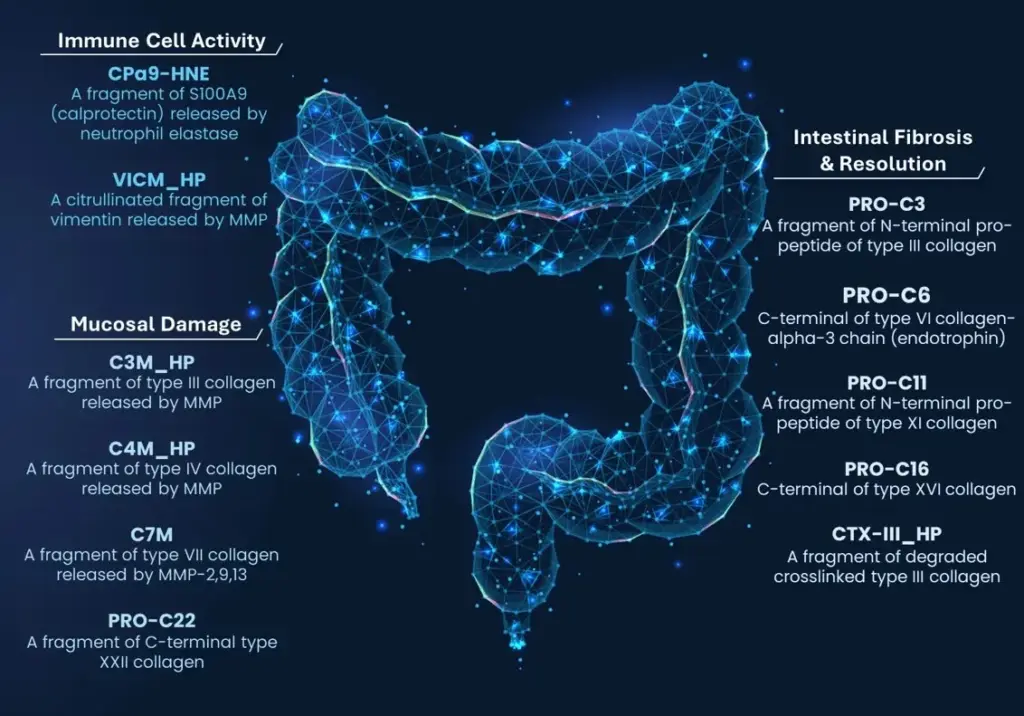
In our recently published paper “Circulating Extracellular Matrix Products as Indicators of Disease Burden and Predictors of Disease Course in Ulcerative Colitis”, we identified C7M (MMP-9/13-degraded type VII collagen α1-chain), nordicPRO-C3™ (type III collagen formation), and PRO-C22 (turnover of type XXII collagen) as biomarkers of endoscopic disease severity and treatment response in UC.
We found that a combination of nordicPRO-C3™ and PRO-C22 predicted endoscopic outcomes at week 24, while lower levels of C7M and the C3M/nordicPRO-C3™ ratio were associated with endoscopic response. Notably, this is the first time that MMP-mediated type VII collagen degradation has been linked to disease severity in UC, highlighting its potential role in epithelial damage and barrier dysfunction.
These biomarkers provide a non-invasive means to assess transmural disease activity and disease extent, addressing a critical gap in disease burden monitoring. Incorporating these biomarkers into clinical practice could improve UC management by refining diagnostic accuracy for predicting endoscopic response prior to treatment adjustment.
Article: Circulating Extracellular Matrix Products as Indicators of Disease Burden and Predictors of Disease Course in Ulcerative Colitis