NordicPRO-C3™ (Lab services)
PopularA neo-epitope fragment of type III collagen released during extracellular matrix remodeling, enabling non-invasive quantification of active fibrogenesis and dynamic assessment of fibrosis progression and treatment response.
A research use only (RUO) assay, measured in our CAP/CLIA-certified lab as an exploratory endpoint or pharmacodynamic biomarker in phase I through phase IV clinical trials. It is available as part of our biomarker testing services and not for diagnostic use.
Key features and values
- Pharmacodynamic biomarker that measures active fibrosis via type III collagen formation
- Monitors treatment response and confirms mechanism of action in clinical trials
- Tracks changes in fibrosis dynamically over time
- Supports patient selection and boosts trial success by predicting disease progression
- Validated in liver diseases including MAFLD and alcohol-related fibrosis
- Used in research across cancer, lung, kidney, heart, and skin fibrosis
- FDA-supported use in tumor fibrosis and fibrotic disease research
- Accelerates drug development by enhancing clinical trial decision-making
Description
At Nordic Bioscience, our approach to measurement is rooted in decades of innovation and a deep understanding of fibrosis. As the originators of PRO‑C3, we perfected the biomarker over 15+ years of clinical trials, built on more than 30 years of expertise in extracellular matrix biomarkers. Today, PRO-C3/nordicPRO‑C3™ is a household name known to ensure you receive data that is both precise and actionable.
As an efficacy and pharmacodynamic biomarker, nordicPRO‑C3™ (PRO-C3) is widely used in clinical trials to monitor treatment response, demonstrate target engagement and confirm mechanism of action. In addition, its dynamic nature makes it ideal for tracking changes in fibrosis over time.
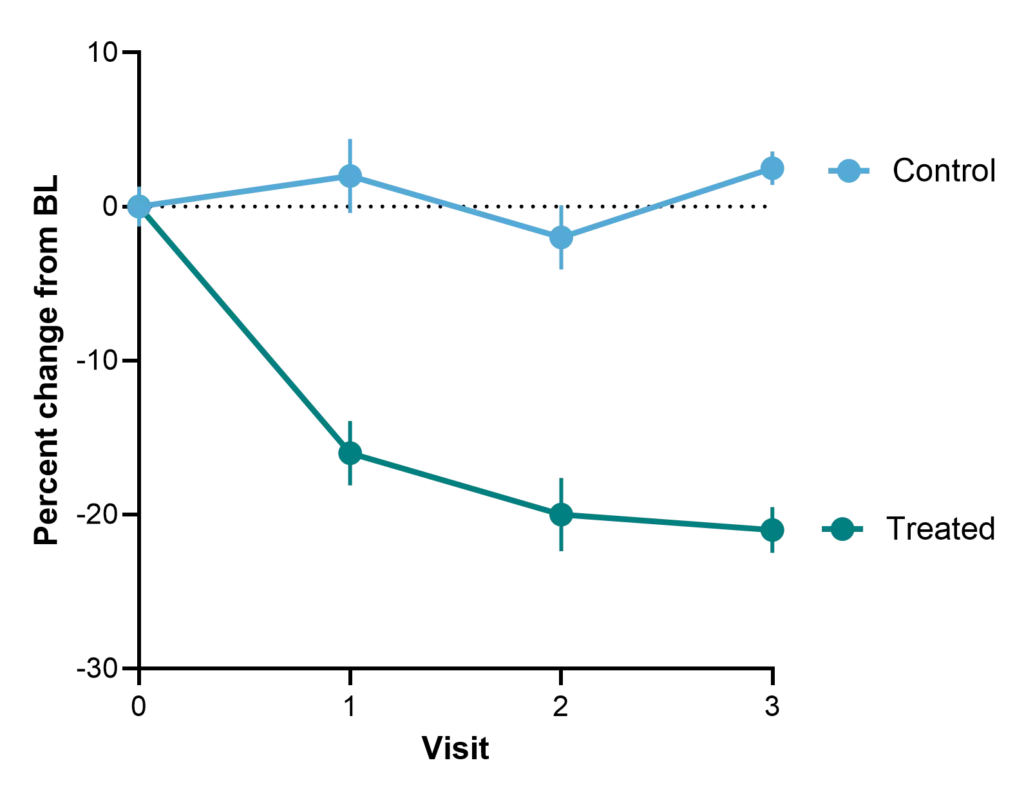
As a prognostic biomarker, nordicPRO-C3™ supports patient selection by identifying individuals more likely to experience disease progression or clinical outcomes — helping enrich trial populations, boost statistical power, and increase the likelihood of success.
NordicPRO C3™ has demonstrated value in liver diseases such as metabolic-associated fatty liver disease (MAFLD), and alcohol-related liver fibrosis, while also predicting disease progression and clinical outcomes. Beyond liver disease, nordicPRO-C3™ is widely used in research on lung fibrosis, tumor fibrosis (FDA-supported), and other conditions where fibrosis plays a central role.
NordicPRO-C3™ provides a powerful tool for monitoring treatment efficacy and tracking disease progression over time. Furthermore, it serves as a critical decision-making tool in clinical trials, helping researchers assess treatment impact, understand the mechanism of action, and accelerate drug development.
We focus on your research needs:
- Our nordicPRO‑C3™ assay is proven and validated over hundreds of clinical trials and is trusted by leading global pharmaceutical partners.
- Operated in a CAP/CLIA‑certified, ISO‑compliant laboratory, our methods have delivered over 120,000 PRO‑C3 results, providing a robust foundation for clinical decision‑making, offering clinical and regulatory excellence.
- With a rapid 24‑hour turnaround and an optimized sample requirement of just 60 µL, we provide operational efficiency. Our workflow is designed to support timely and efficient research.
- Our exclusive ProteinFingerPrint Technology™ turns data into insight. We transform complex collagen turnover data into clear insights, empowering you to improve patient selection, monitor treatment efficacy, and drive precision in fibrosis research.
Disclaimer: This biomarker is for research use only. It is not intended for use in diagnostic procedures or therapeutic interventions.
Patient selection for more efficient clinical trails
Clinical trials are a critical and costly step in drug development. Reducing trial size while maintaining robust data is essential for accelerating market entry and managing costs.
NordicPRO‑C3™ transforms clinical trial efficiency by enabling precise pre-stratification of patients. By identifying individuals with active fibrosis who are most likely to respond to treatment, nordicPRO‑C3™ helps optimize patient selection, ensuring trials enroll the right participants from the start. This not only enhances trial success rates but also significantly reduces the number of enrollees needed—saving time, resources, and costs while increasing confidence in the therapeutic’s efficacy.
By integrating nordicPRO‑C3™ into clinical trial design, drug developers can streamline recruitment, improve study outcomes, and bring innovative fibrosis-targeted therapies to market faster.
Actionable insights into drug mode of action
Understanding a drug’s precise mode of action (MOA) is critical for evaluating safety, optimizing efficacy, and advancing precision medicine. However, in many cases, the exact MOA remains unclear.
NordicPRO‑C3™ provides valuable insights into MOA by detecting active fibrosis formation. A key process in disease progression and treatment response. By integrating nordicPRO‑C3™ into clinical trials, researchers gain a deeper understanding of how a drug interacts with fibrotic pathways, supporting data-driven decisions on safety, efficacy, and patient stratification.
Prediction of drug response
Not all patients respond equally to treatment—many experience varying levels of efficacy and safety, while some fail to respond at all. This leads to wasted resources, ineffective care, and increased healthcare costs.
NordicPRO‑C3™ changes this by providing a powerful tool to predict treatment response in fibrotic diseases. By measuring active fibrosis, nordicPRO‑C3™ helps identify patients who are most likely to benefit from specific therapies. This enables clinicians to make informed treatment decisions, reduces unnecessary treatments, and supports reimbursement strategies.
Monitoring treatment efficacy and pharmacodynamics
Effective drug development requires real-time insights into treatment efficacy and pharmacodynamic effects to ensure therapies are working as intended. Traditional endpoints often fail to capture early dynamic changes in fibrosis, delaying crucial adjustments in clinical strategies.
nordicPRO‑C3™ offers a dynamic solution by enabling longitudinal monitoring of active fibrosis remodeling, providing direct evidence of a drug’s impact on fibrotic pathways already in phase IB. By tracking changes in nordicPRO‑C3™ levels over time, researchers can assess whether a therapy is effectively reducing fibrosis, determine optimal dosing strategies, and detect early signs of treatment response.
By integrating nordicPRO‑C3™ into clinical trials and treatment protocols, drug developers and clinicians gain a powerful pharmacodynamic biomarker that validates drug efficacy, supports regulatory decision-making, and accelerates the path to precision fibrosis therapies.
Which diseases can NordicPRO‑C3™ be used in?
-
- Idiopathic Pulmonary Fibrosis (IPF)
- Systemic Sclerosis-Associated Interstitial Lung Disease (SSc-ILD)
- Chronic Obstructive Pulmonary Disease (COPD) with fibrosis
-
- Heart failure with fibrosis (HFpEF, HFrEF)
- Hypertensive heart disease with fibrosis
-
- Pancreatic cancer
- Breast cancer
- Lung cancer
- Colorectal cancer
- Melanoma
Publications
Collagen Type III and VI Turnover in Response to Long-Term Immobilization
Performance of the PRO-C3 collagen neo-epitope biomarker in non-alcoholic fatty liver disease




