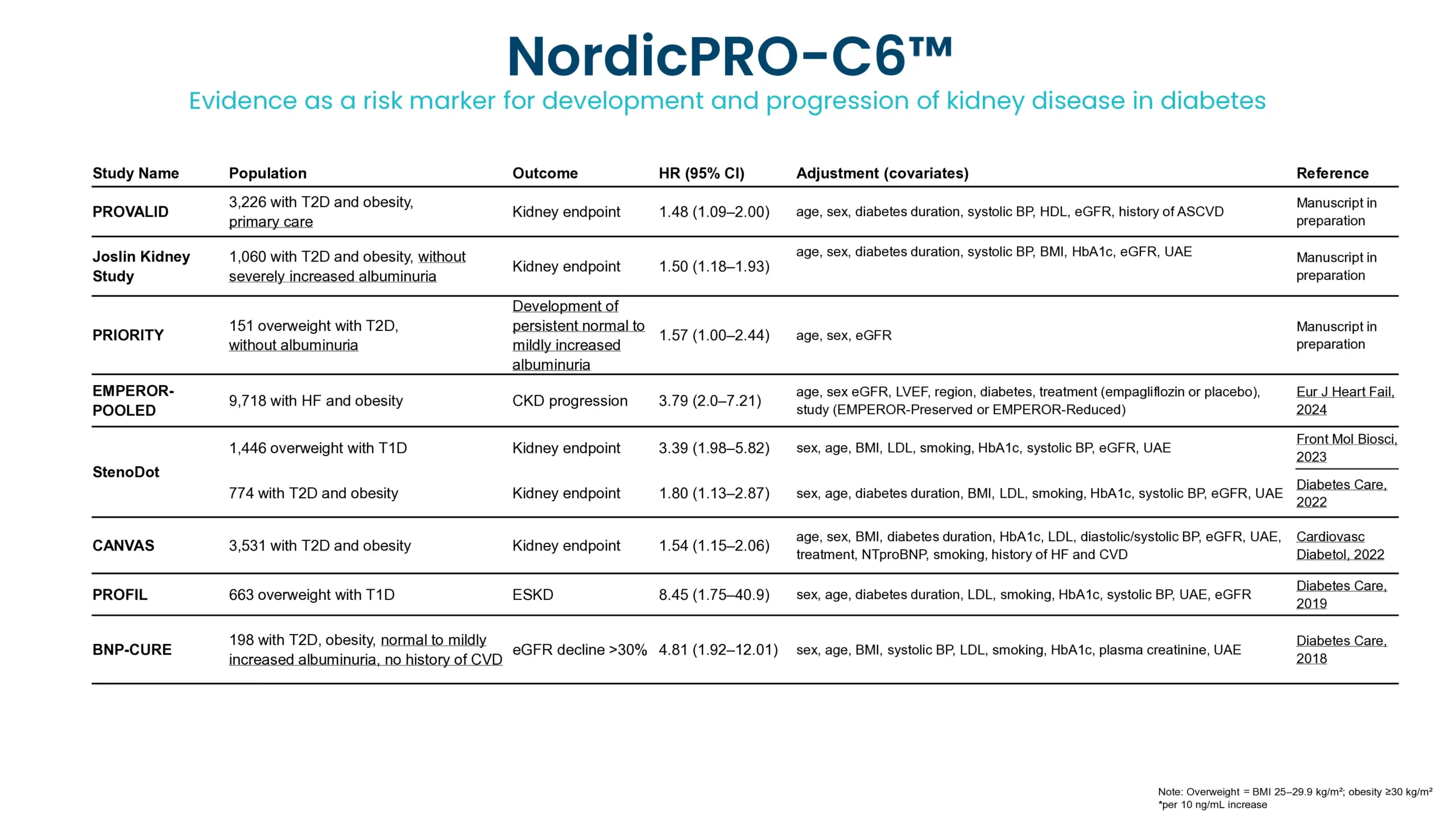
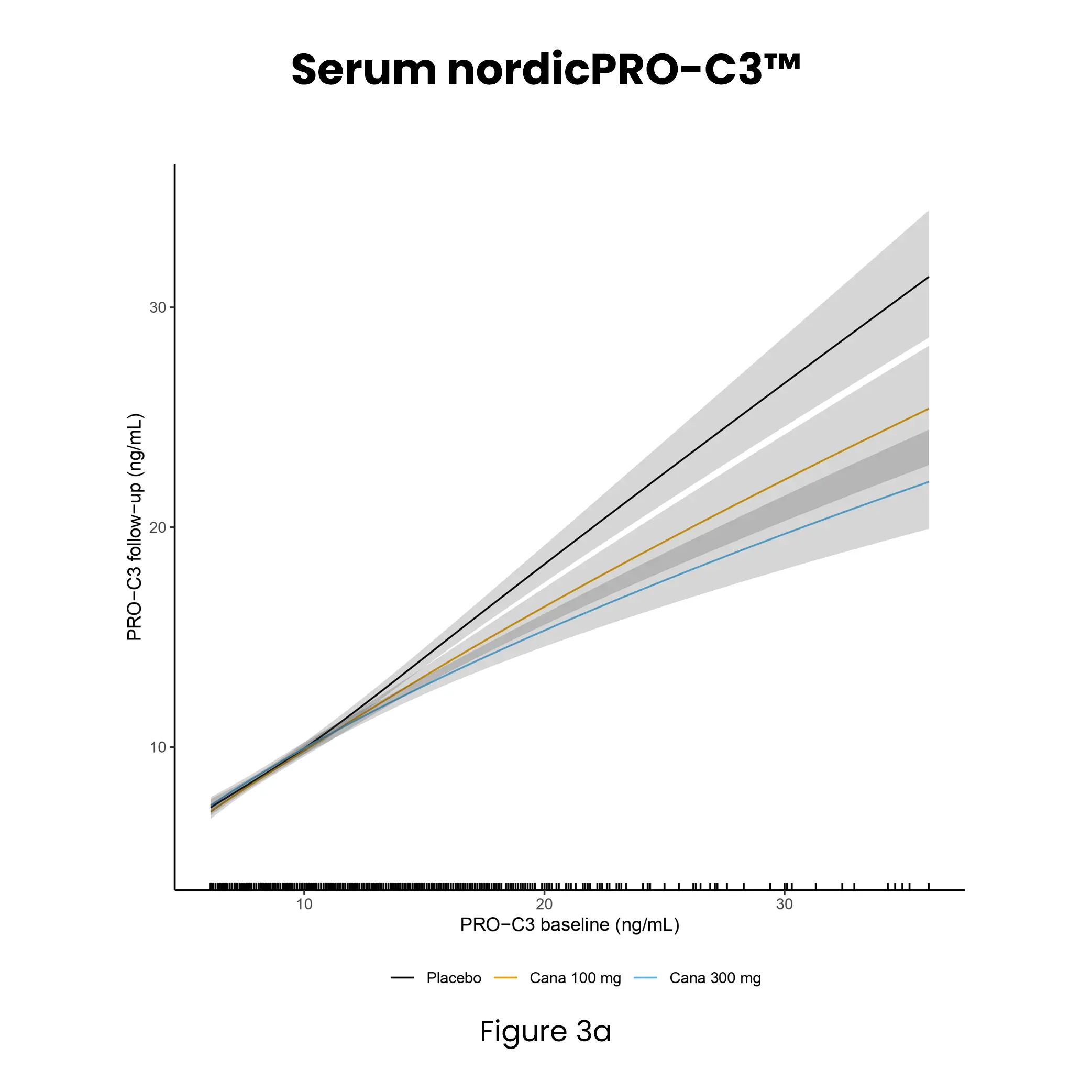
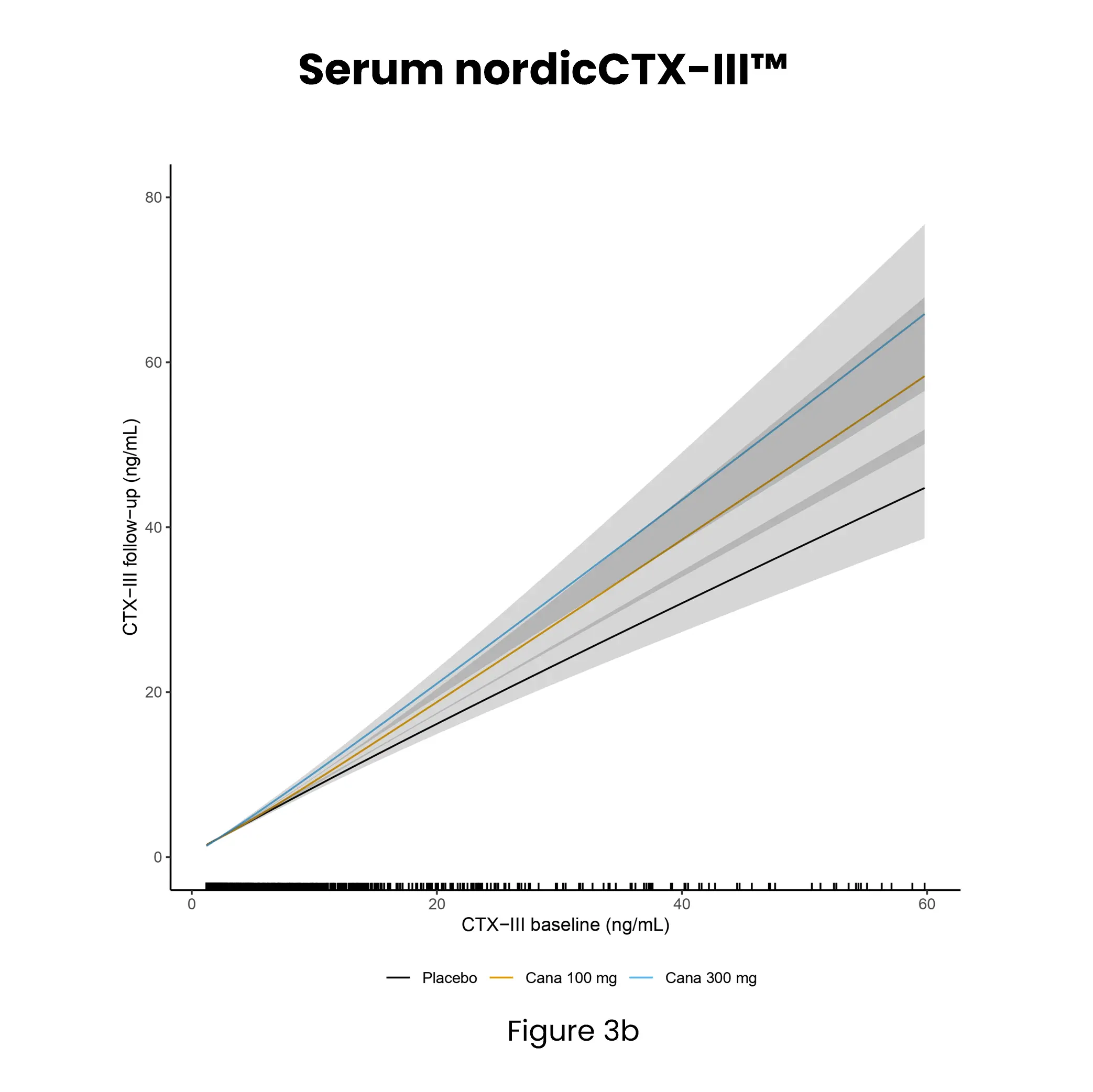
Diabetic kidney disease (DKD) is a major complication of diabetes and can progress to kidney failure. Despite advances in treatment, many patients continue to progress due to ongoing, often subclinical, fibrotic injury in the kidney. Conventional clinical markers such as albuminuria and eGFR are limited in detecting early fibrotic changes or reflecting treatment effects.
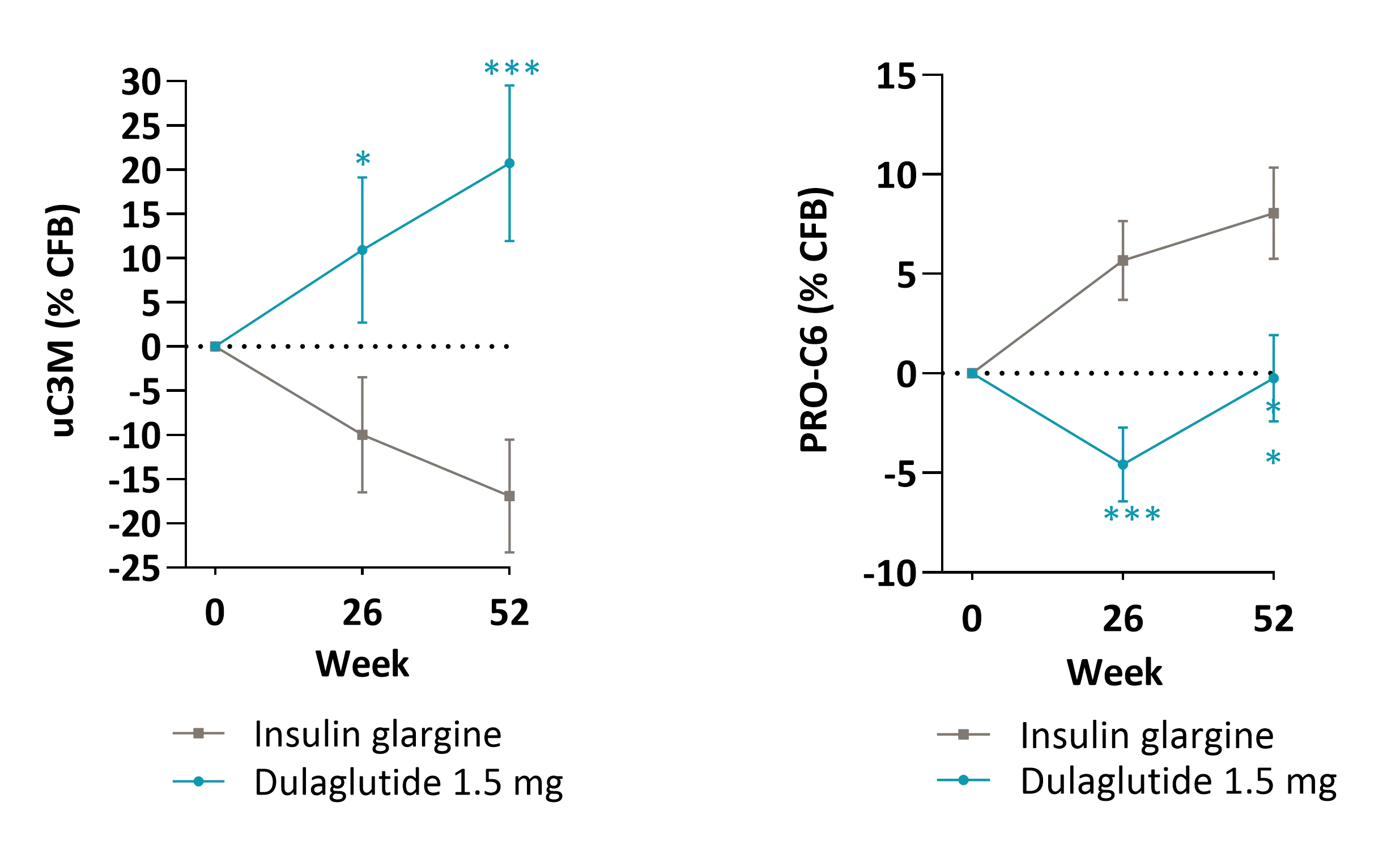
Kidney fibrosis, a hallmark of DKD, is driven by extracellular matrix (ECM) accumulation and remodeling, which contributes to declining kidney function. ECM-derived biomarkers offer direct insight into active fibrogenesis and fibrolysis, enabling earlier detection of disease progression.