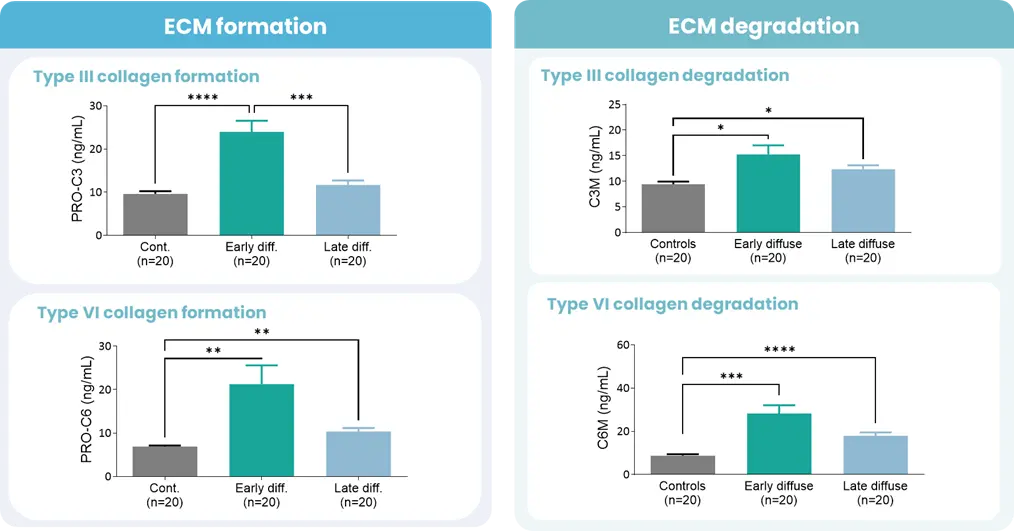
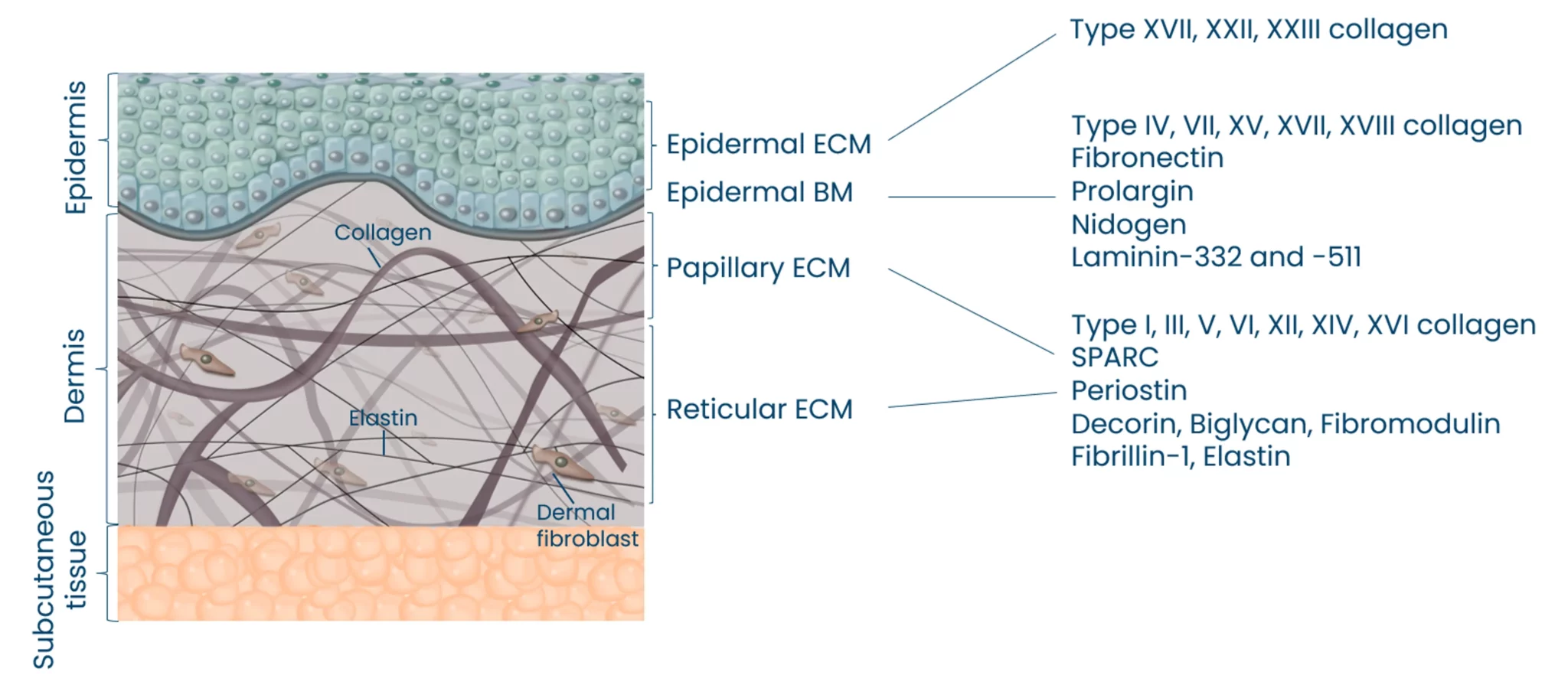
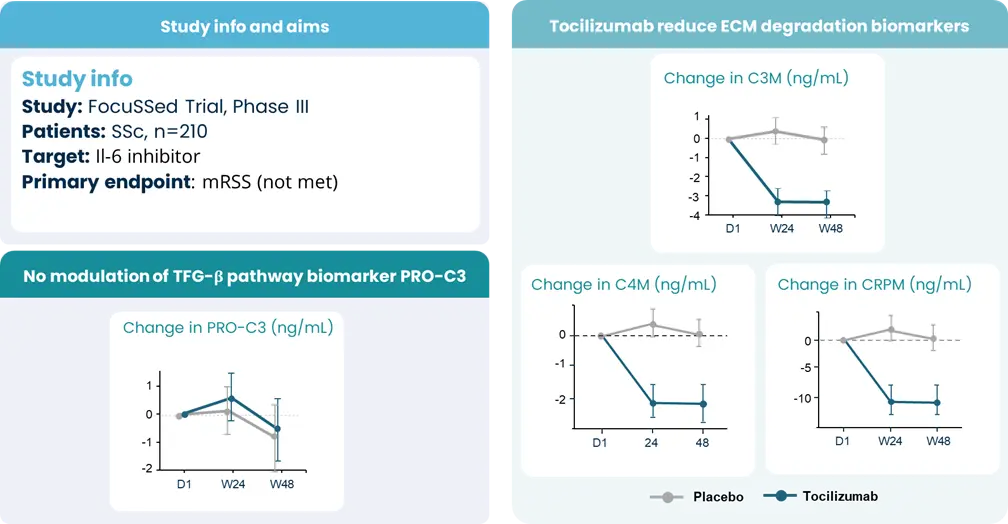
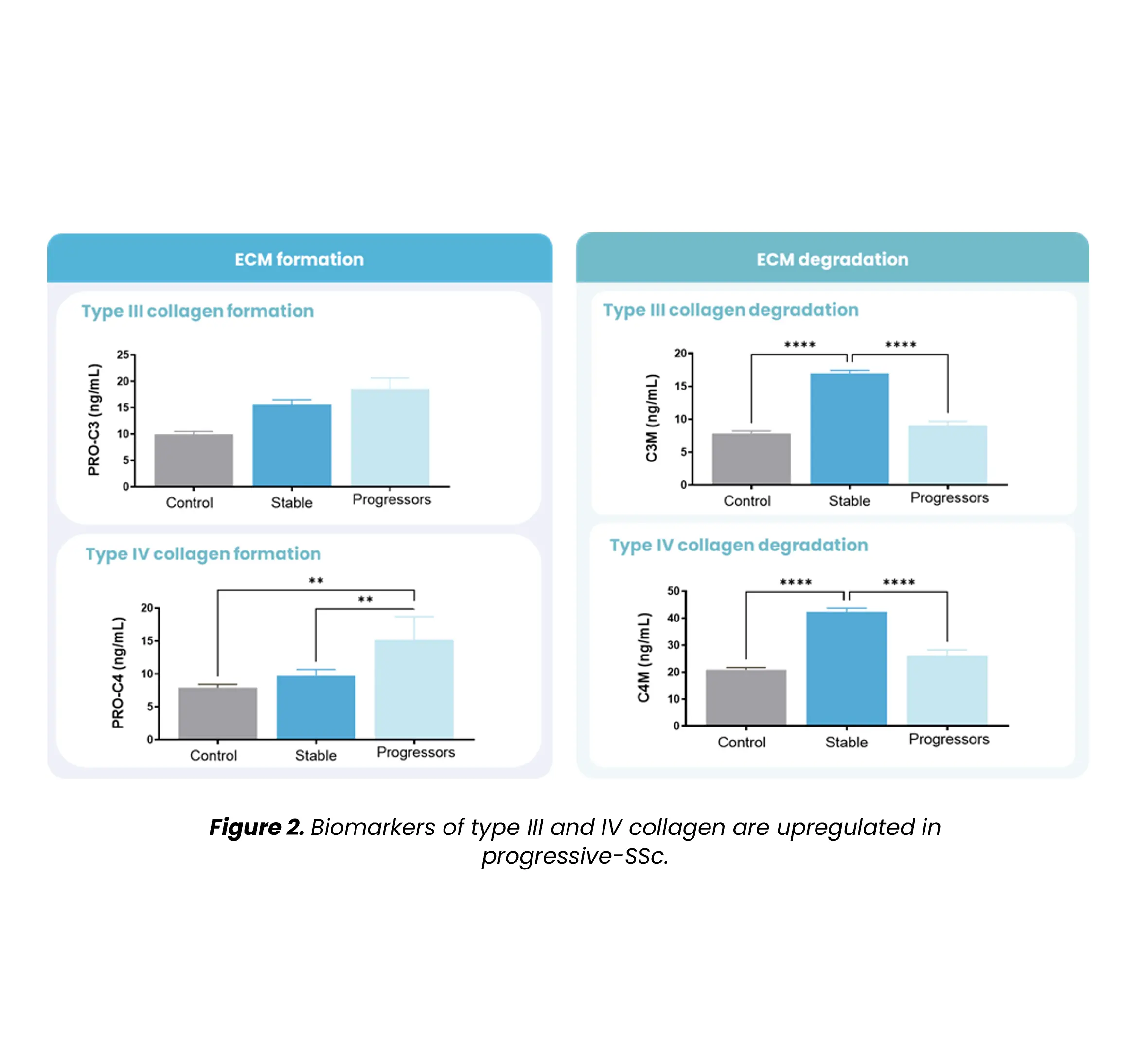
Autoimmune diseases, such as scleroderma—systemic sclerosis (SSc)—affect several tissues, leading to fibrotic tissue buildup of the skin and internal organs. Fibrosis is caused by an increased synthesis of extracellular matrix (ECM), which plays a key role in SSc pathogenesis.
Did you know?
Extracellular matrix-specific biomarkers that help us quantify and monitor fibrosis of the skin internal organs are key when we want to understand the disease mechanisms and prediction of scleroderma/SSc progression. Utilizing biomarkers can help address several aspects of unmet needs in SSc. A well-designed biomarker program enables precise diagnosis, prediction of clinical course and organ involvement, evaluation of therapeutic responsiveness, and identification of novel therapeutic targets.