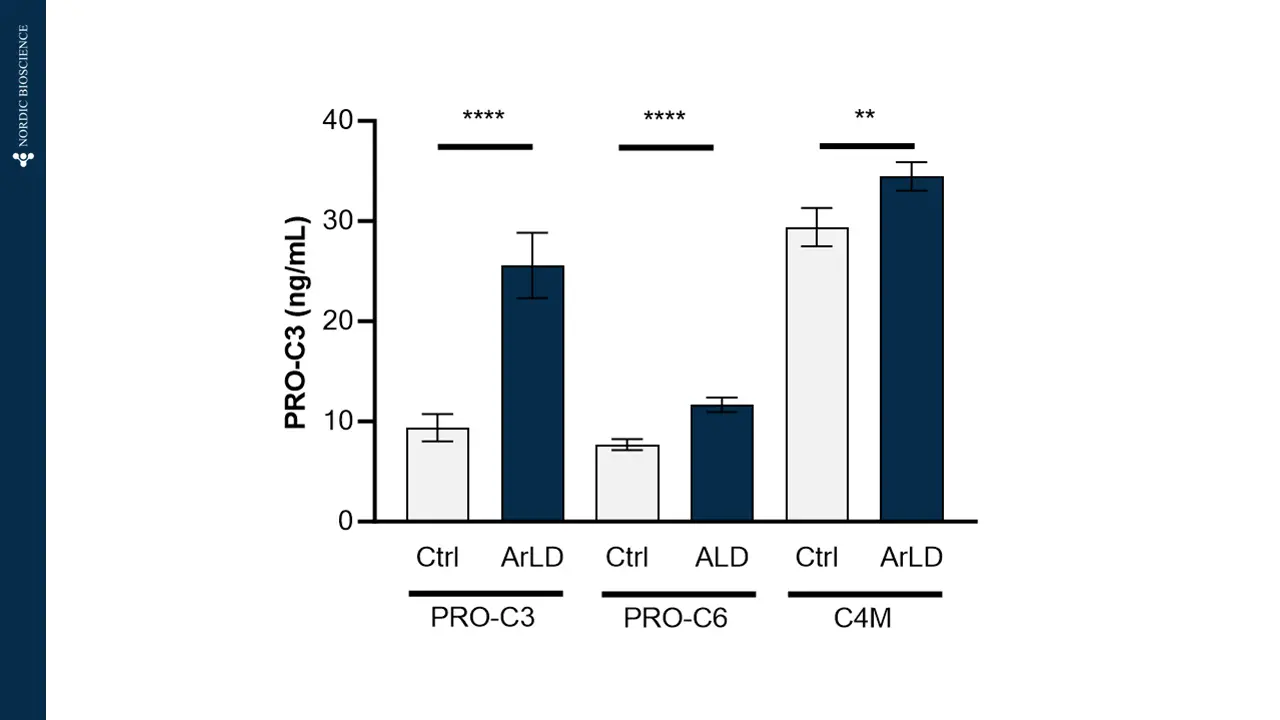
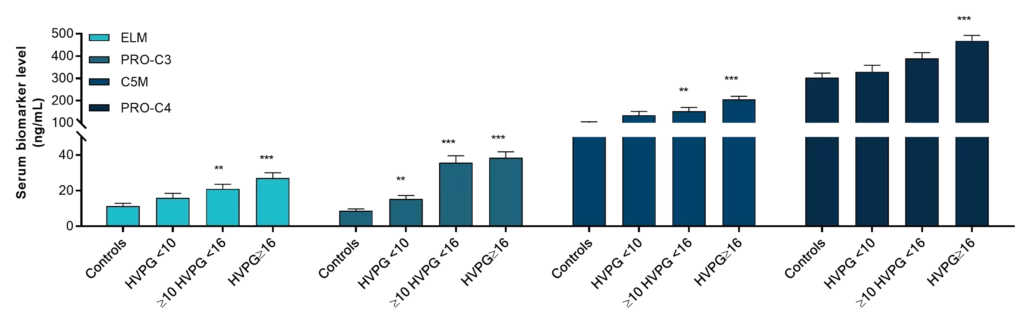
The development and increase in liver fibrosis is a key component of alcohol-related liver disease (ALD), formerly known as alcoholic liver disease (ALD). This progression can result in cirrhosis or further develop into hepatocellular carcinoma and increased mortality. Patient monitoring using fibrosis biomarkers in ALD patients represents a potential novel diagnostic and disease management tool.
Nordic Bioscience’s non-invasive biomarkers based on extracellular matrix (ECM) remodeling can help to overcome the challenges in diagnosing ALD and finding patients at high risk of disease progression. Furthermore, the use of predictive or prognostic biomarkers of the ECM to enrich or stratify patients likely to respond to a therapeutic in drug development trials may reduce the trial length, and size required to determine therapeutic efficacy.