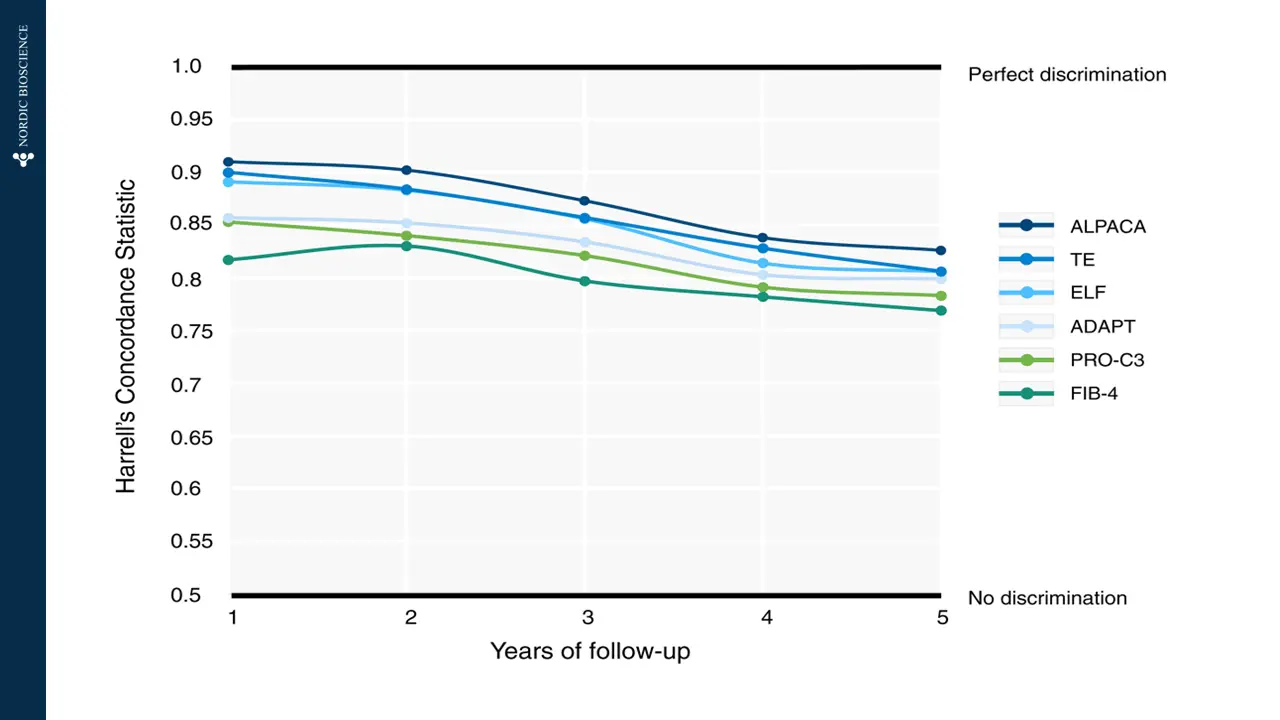
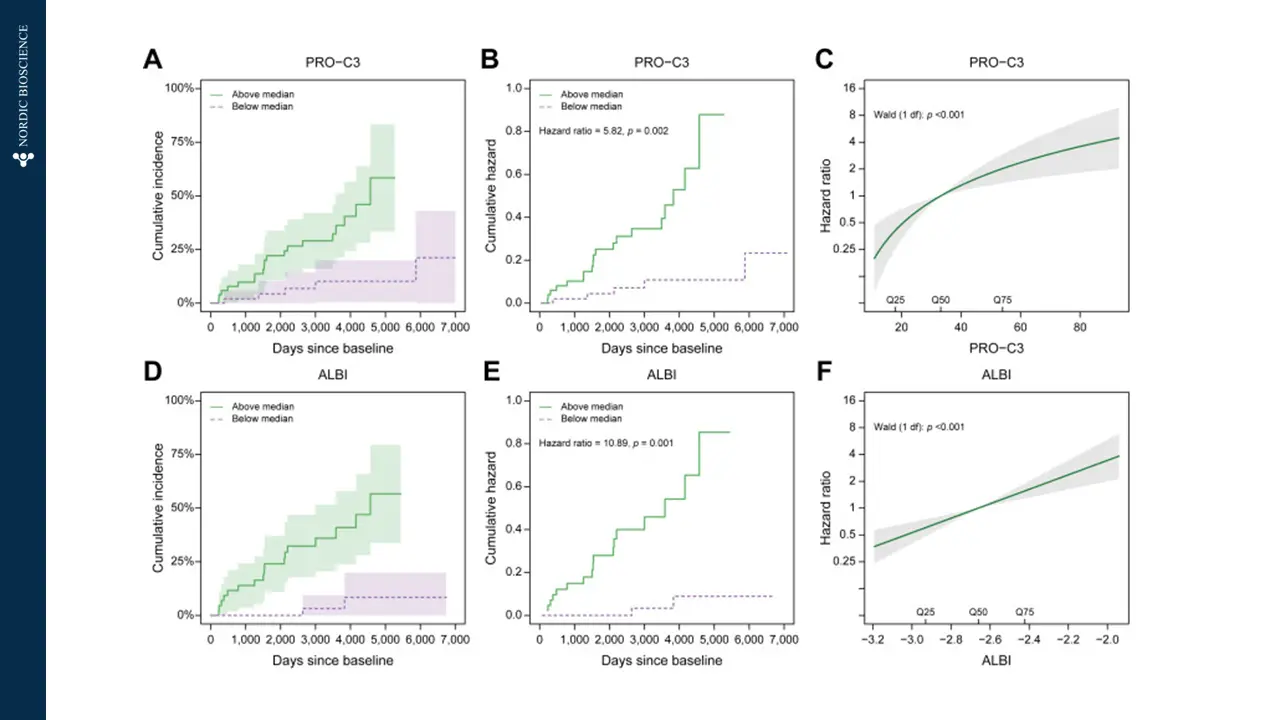
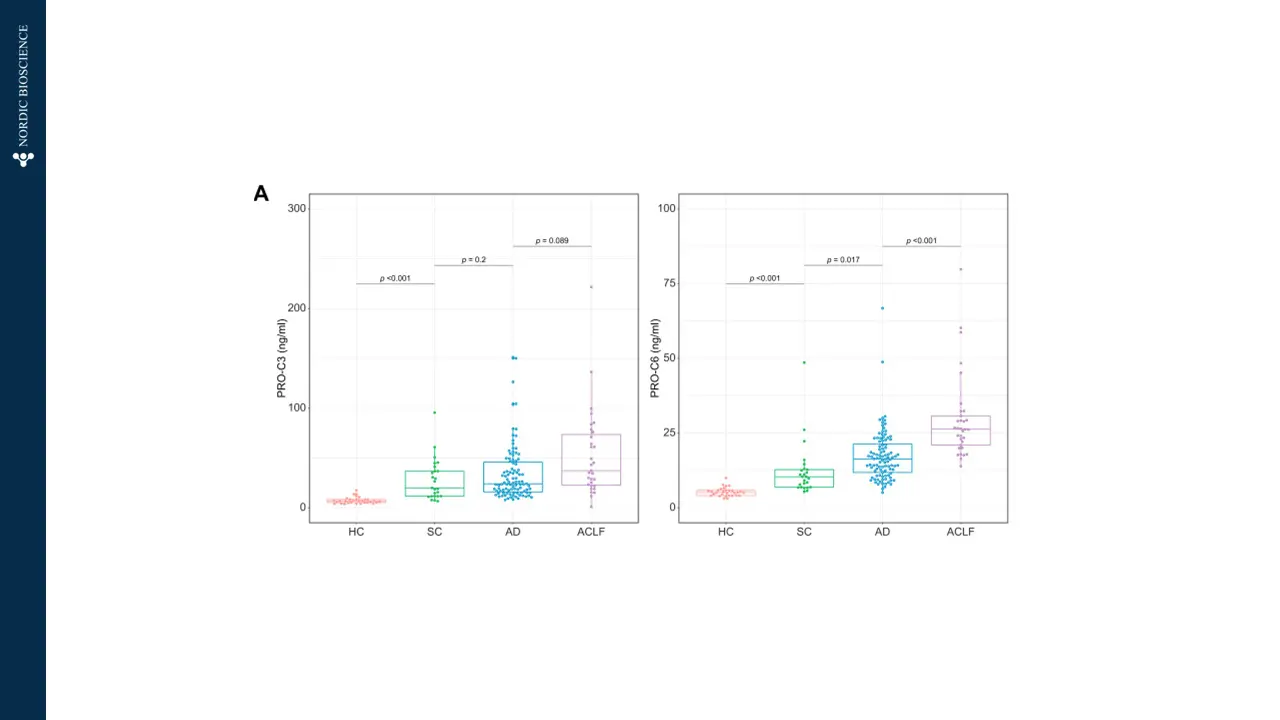
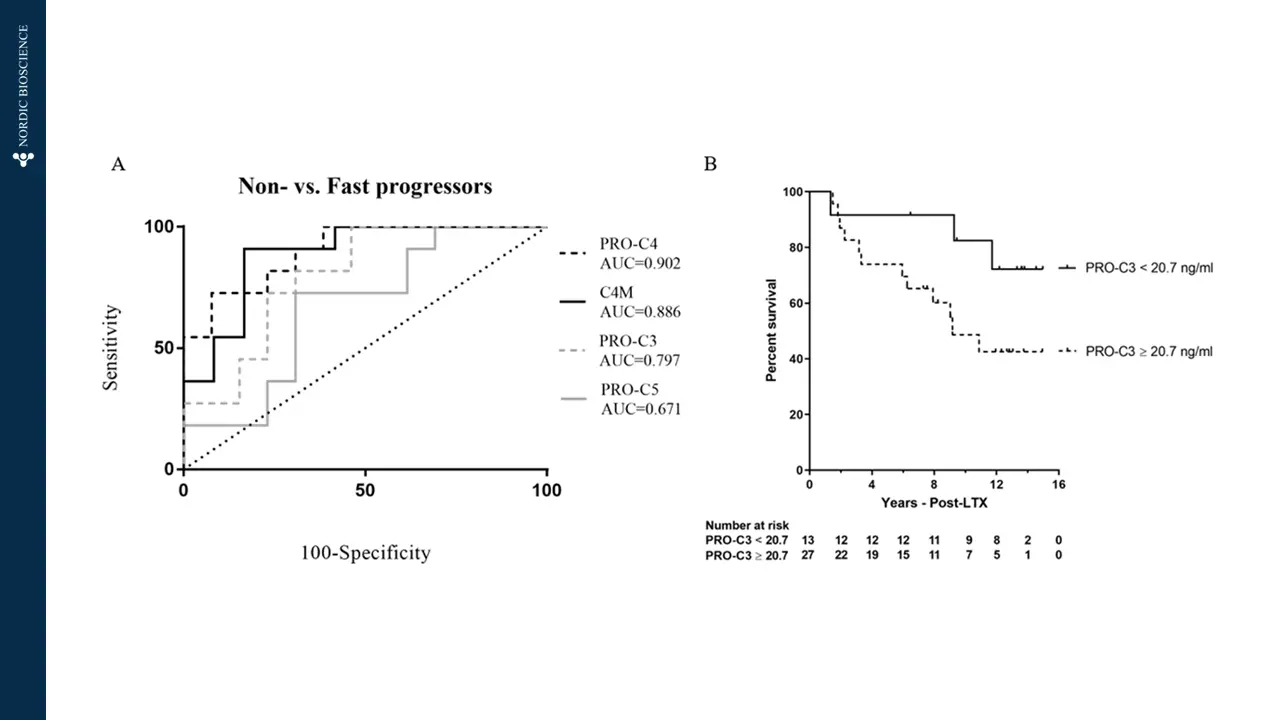
The current diagnostic methods for end-stage liver disease and cirrhosis often involve invasive procedures and may not offer timely results. Blood-based biomarkers can provide tools to offer immediate treatment to patients with liver failure and identify the potential need for liver transplant by enabling early detection and intervention, leading to improved patient outcomes. Such biomarkers could significantly enhance the management and care of patients with chronic liver conditions, offering hope for a better quality of life and reducing the burden on healthcare systems.
Nordic Bioscience’s biomarkers are designed to specifically target the dynamics of collagen deposition, which is the main cause of liver scarring in cirrhosis. These biomarkers offer multiple benefits, including efficient patient stratification for advanced fibrosis (F3-F4), the ability to monitor drug response and pharmacodynamics during clinical trials, and the potential to provide valuable prognostic information for clinical outcomes and mortality in patients with end-stage liver disease.