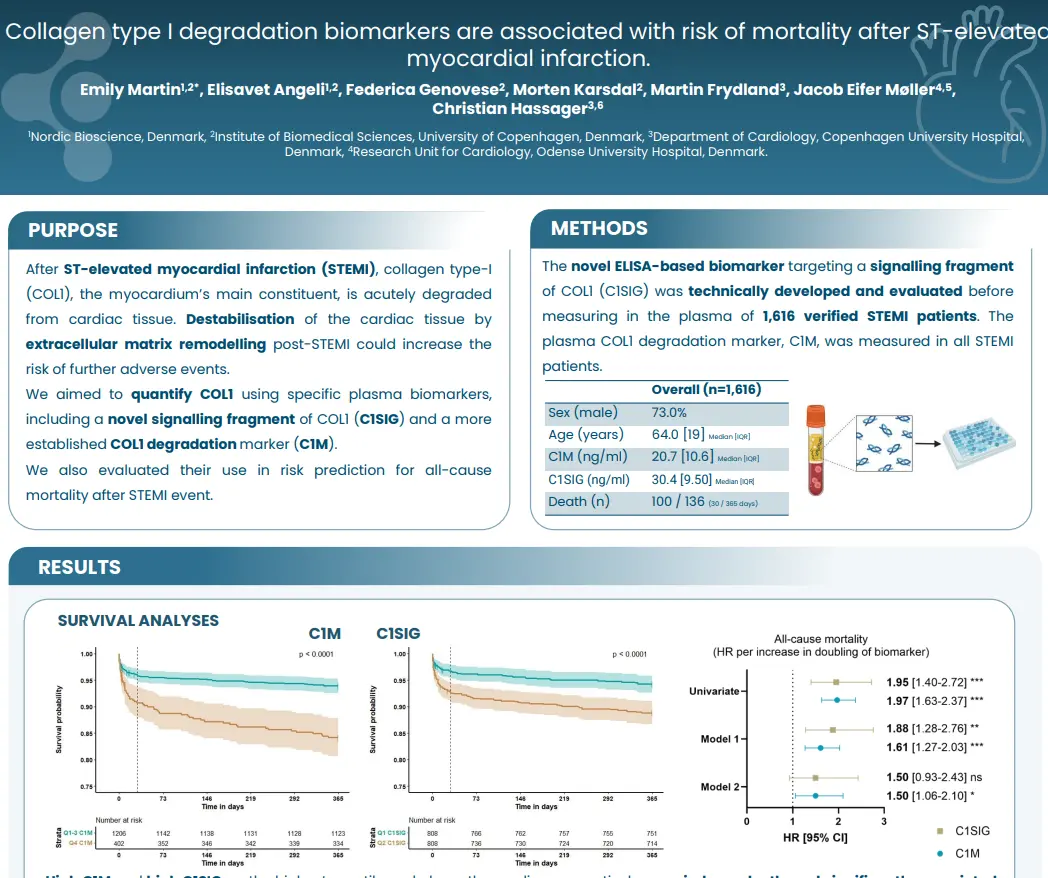
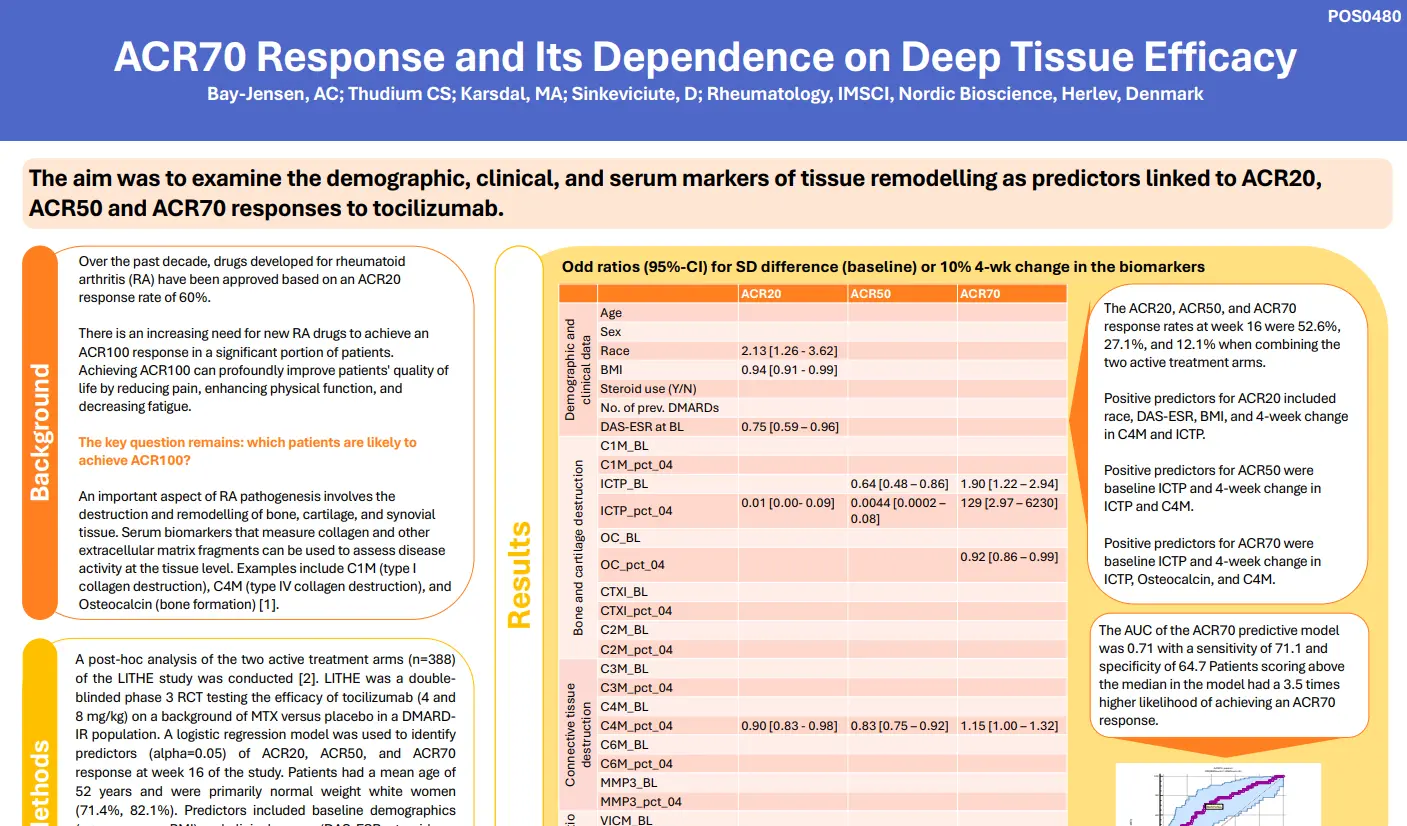
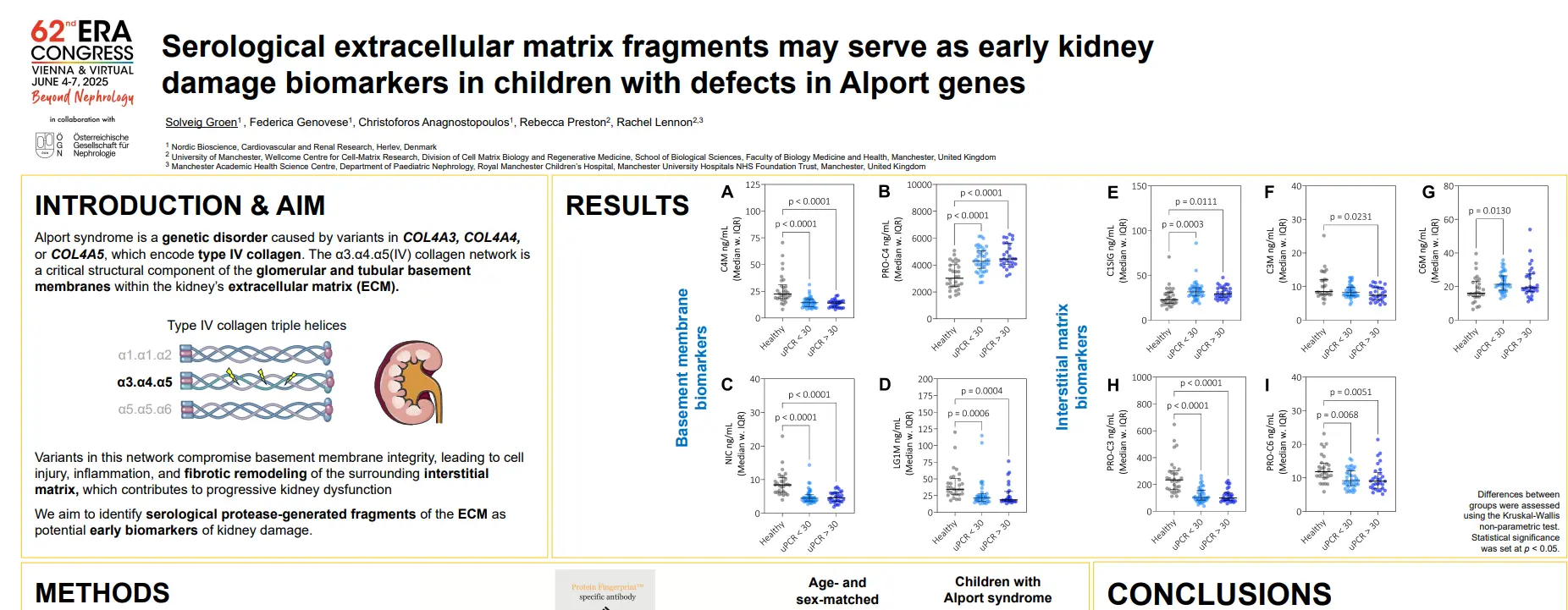
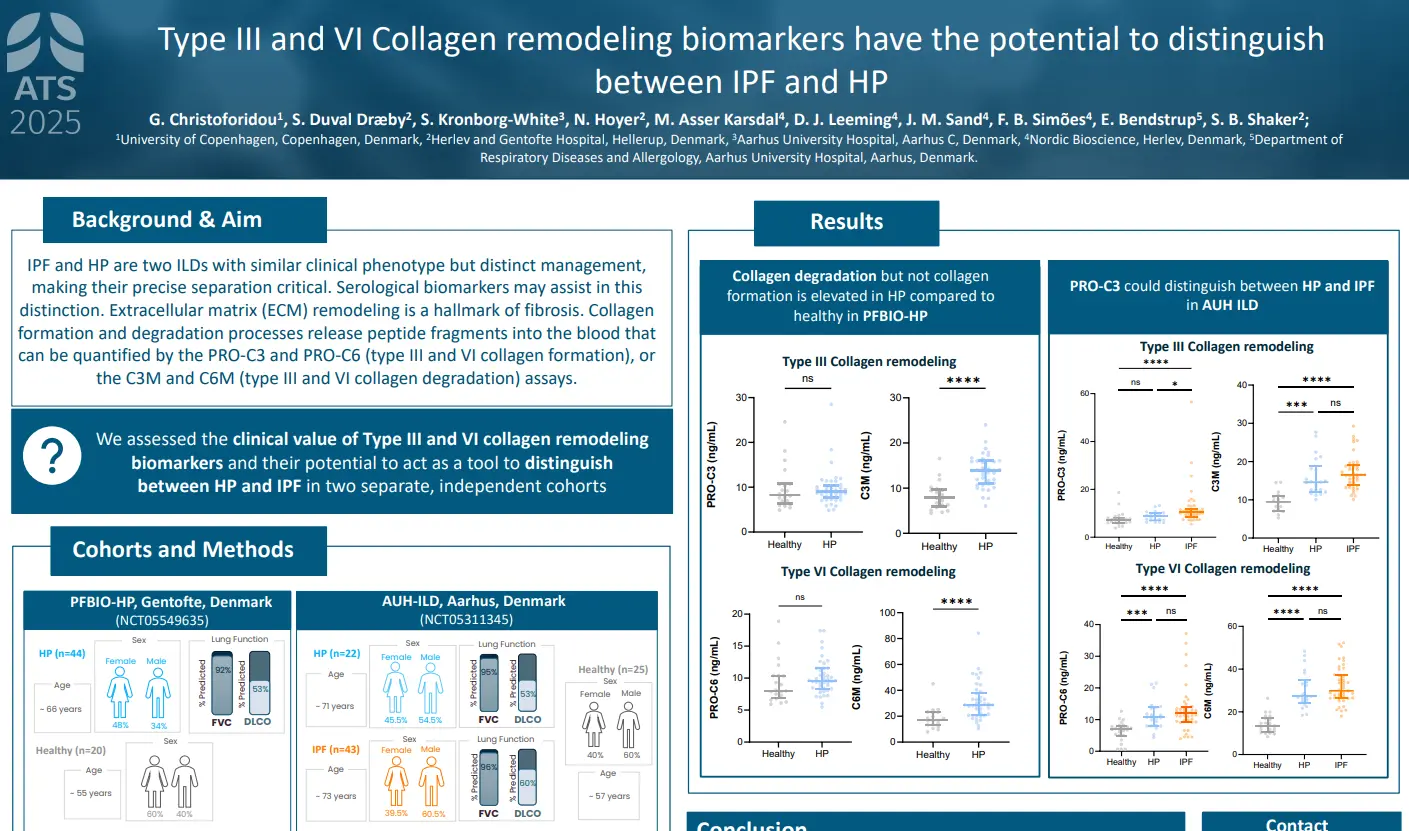
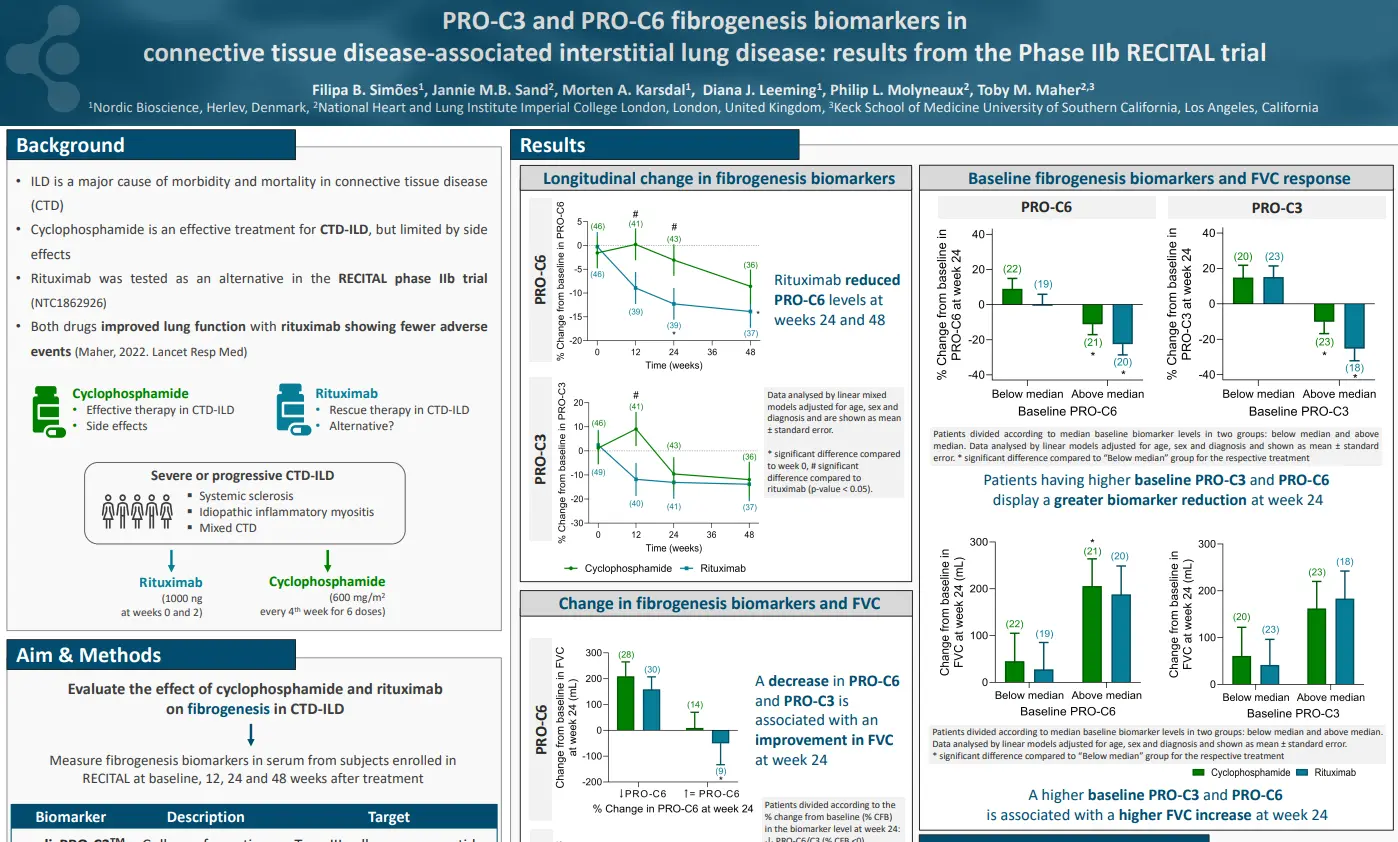
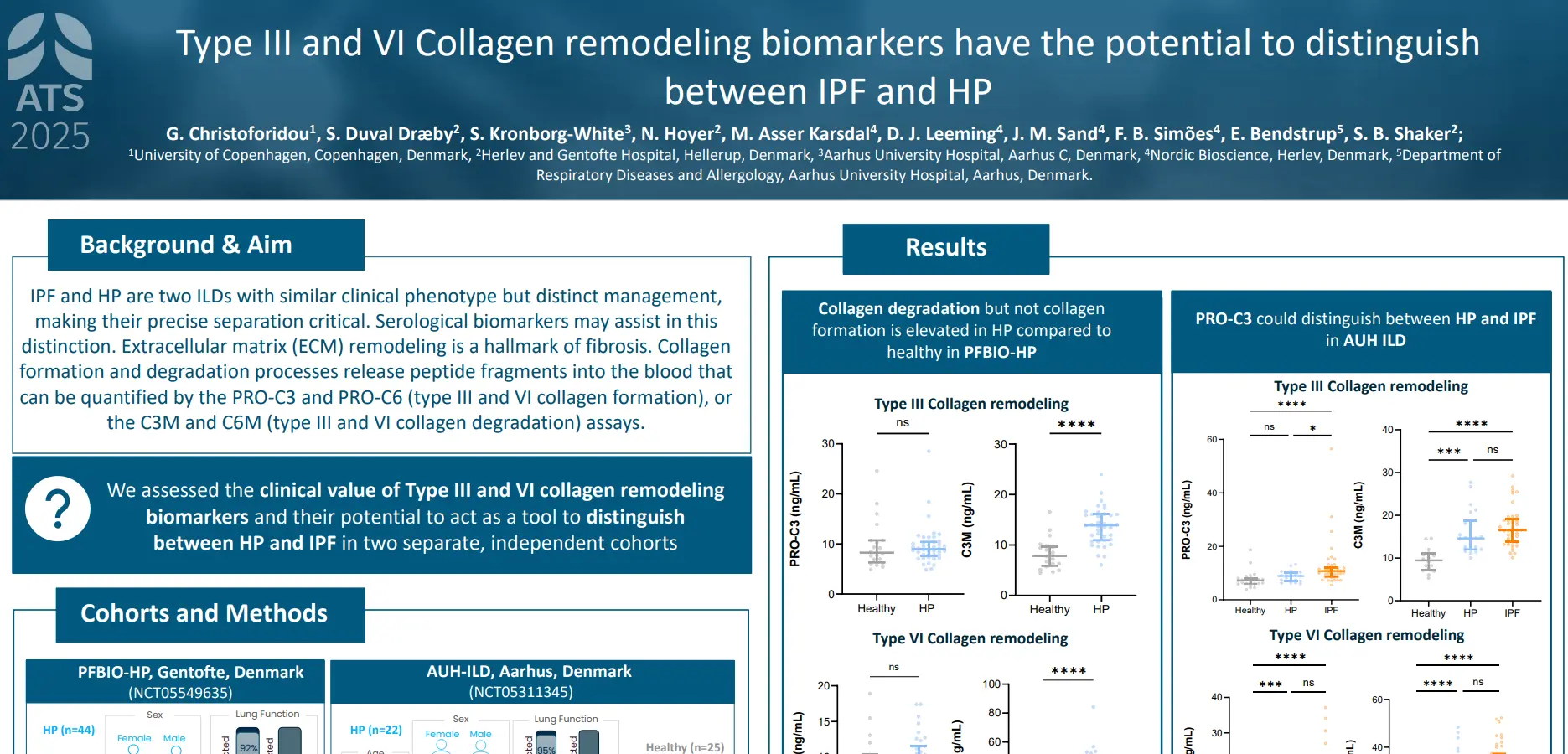
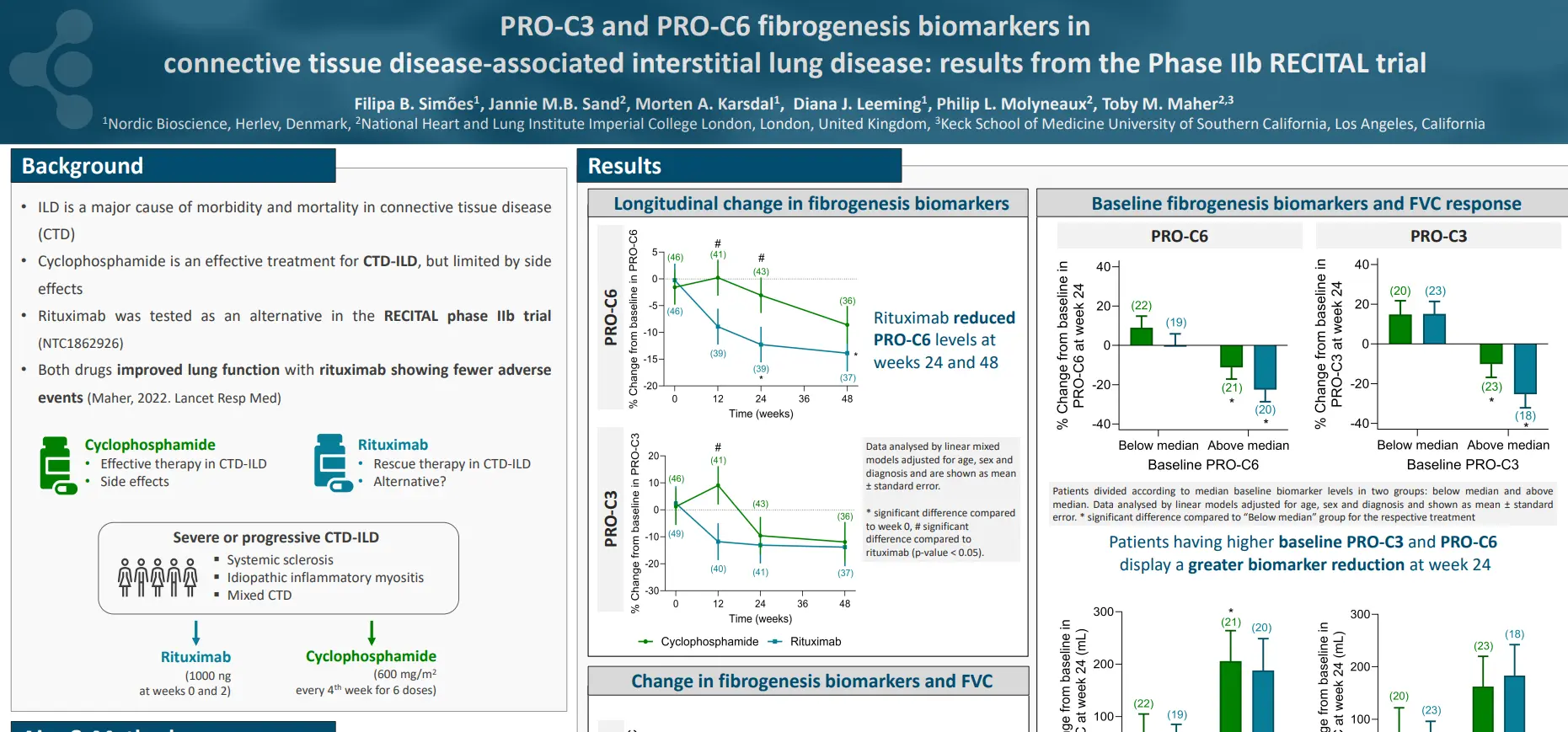
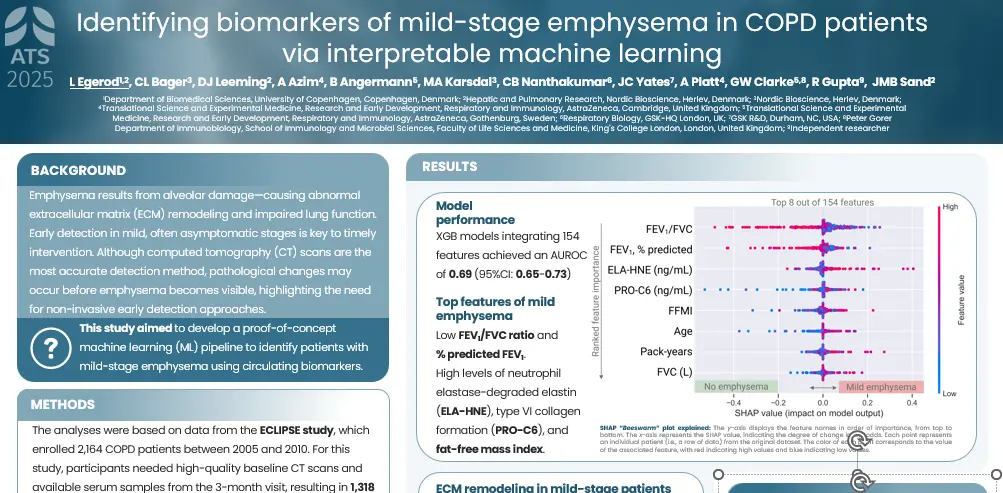
Canstatin, a type IV collagen fragment, is associated with risk of cardiovascular and all-cause mortality in patients with advanced atherosclerosis
Introduction
Atherosclerosis, a common underlying cause of cardiovascular disease, is defined by the formation of
plaques in the arterial walls. Changes in the ECM composition impact the risk for plaque rupture, which may cause acute complications (i.e. stroke or myocardial infarction (MI)). Type IV collagen is primarily known as a major component of basement membranes and has previously been reported to promote plaque stability. Canstatin is the non-collagenous C-terminal domain of type IV collagen alpha 2 chain. It is not only a by-product of proteolytic activity, but also a bioactive molecule. This study investigated if canstatin was associated with adverse outcomes in patients with advanced carotid atherosclerosis.
Poster
Conclusion
Higher circulating canstatin levels in patients undergoing carotid endarterectomy predicted cardiovascular mortality and all-cause mortality over 7.5 years. This suggests that canstatin is a potential novel tool for risk stratification in patients with advanced atherosclerosis, warranting further studies.