Tocilizumab demonstrates superior inhibition of MMP-mediated basement membrane collagen degradation compared to methotrexate or placebo
Introduction
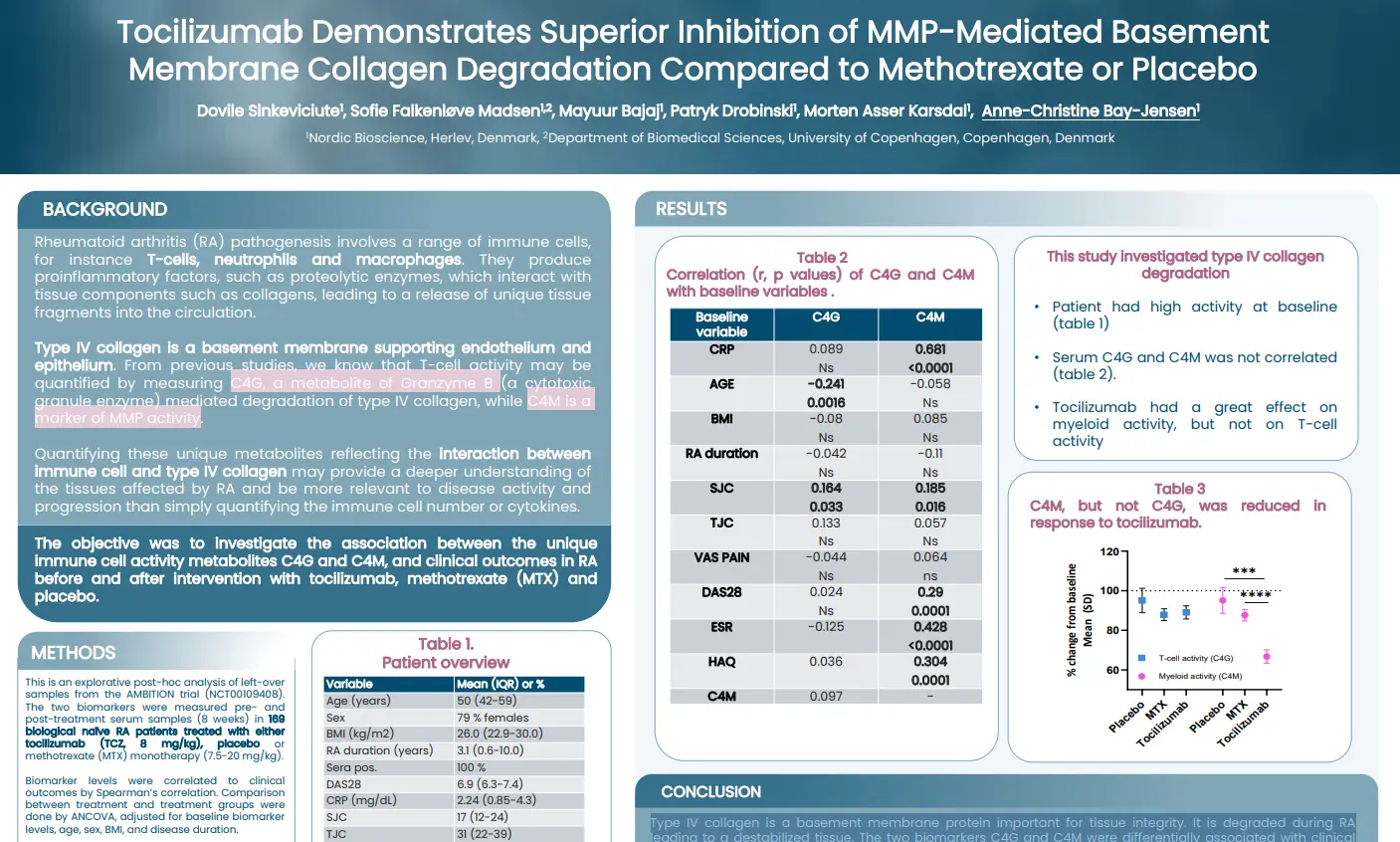
Rheumatoid arthritis (RA) pathogenesis involves a range of immune cells, for instance T-cells, neutrophils and macrophages. They produce proinflammatory factors, such as proteolytic enzymes, which interact with tissue components such as collagens, leading to a release of unique tissue fragments into the circulation. Type IV collagen is a basement membrane supporting endothelium and epithelium. From previous studies, we know that T-cell activity may be quantified by measuring C4G, a metabolite of Granzyme B (a cytotoxic granule enzyme) mediated degradation of type IV collagen, while C4M is a marker of MMP activity. Quantifying these unique metabolites reflecting the interaction between immune cell and type IV collagen may provide a deeper understanding of the tissues affected by RA and be more relevant to disease activity and progression than simply quantifying the immune cell number or cytokines.
The aim of this study was to investigate the association between the unique immune cell activity metabolites C4G and C4M, and clinical outcomes in RA before and after intervention with tocilizumab, methotrexate (MTX) and placebo.
Poster
Conclusion
Type IV collagen is a basement membrane protein important for tissue integrity. It is degraded during RA
leading to a destabilized tissue. The two biomarkers studied, C4G and C4M, were differentially associated with clinical outcome measures. Importantly, only C4M, a marker of MMP-derived tissue destruction, could be inhibited by tocilizumab. None of the markers were modulated by MTX.