Serum biomarkers of proteolytic tissue destruction, formation and macrophage activity can discern patients with IBD according to infliximab treatment non-response or response
Introduction
Characterized by chronic inflammation, patients with Inflammatory Bowel Disease (IBD) experience detrimental remodeling of their intestinal extracellular matrix (ECM). Treatment with anti-inflammatory drugs can reduce inflammation, leading to remission and tissue healing. However, adequate monitoring of patients is critical to ensure and maintain treatment response.
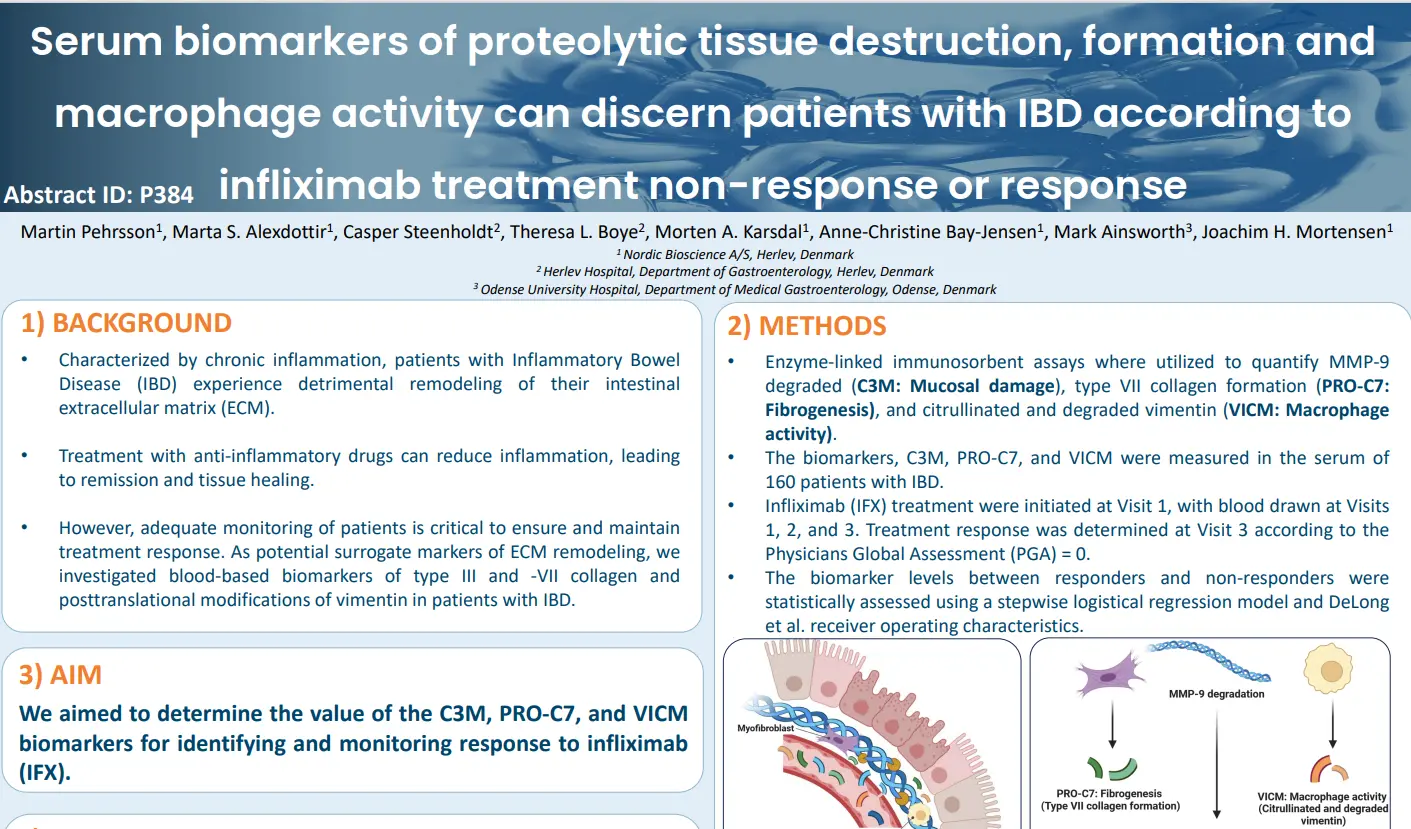
As potential surrogate markers of ECM remodeling, we investigated blood-based biomarkers of type III and -VII collagen and posttranslational modifications of vimentin in patients with IBD. Our aim was to determine the value of the C3M, PRO-C7, and VICM biomarkers for identifying and monitoring response to infliximab (IFX).
Poster
Conclusion
Quantifying a combination of non-invasive biomarkers of ECM remodeling and macrophage activity provided AUCs of 0.684 to 0.797 identifying responders to IFX treatment. Each biomarker provided value at the three different visits (Visit 1, 2, and 3). Combining all three biomarkers measured at each visit
resulted in an AUC of 0.797 identifying responders to IFX treatment.