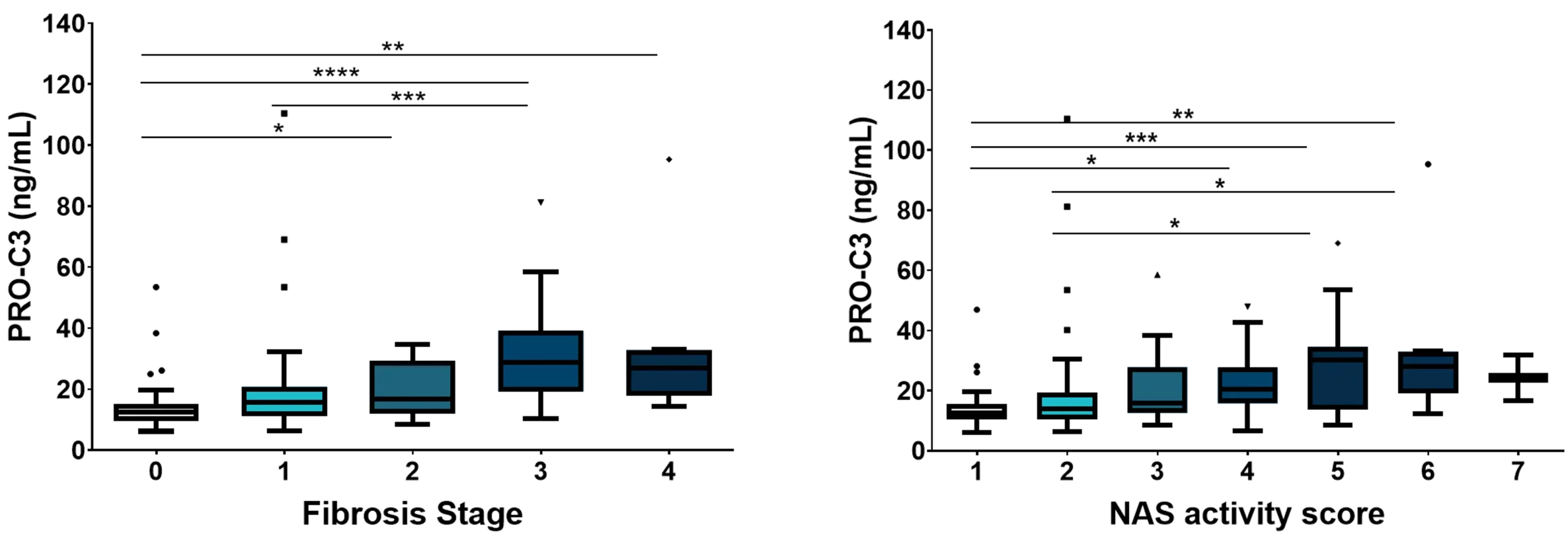
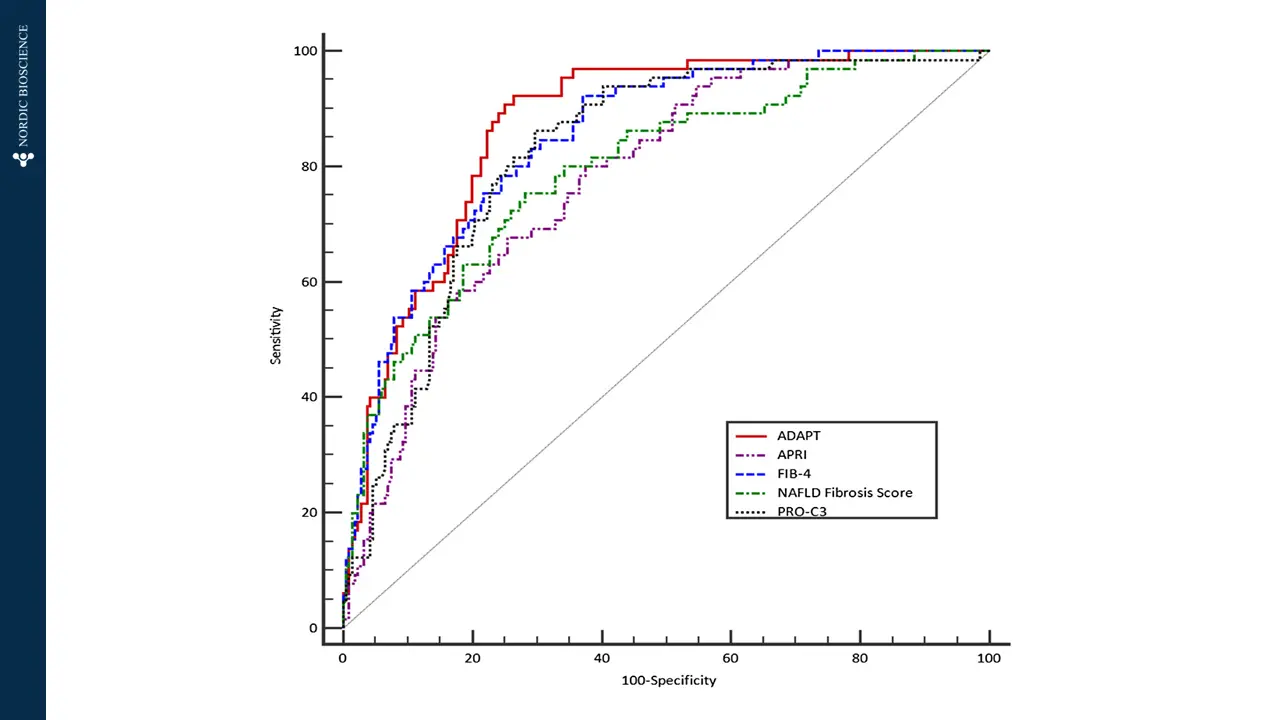
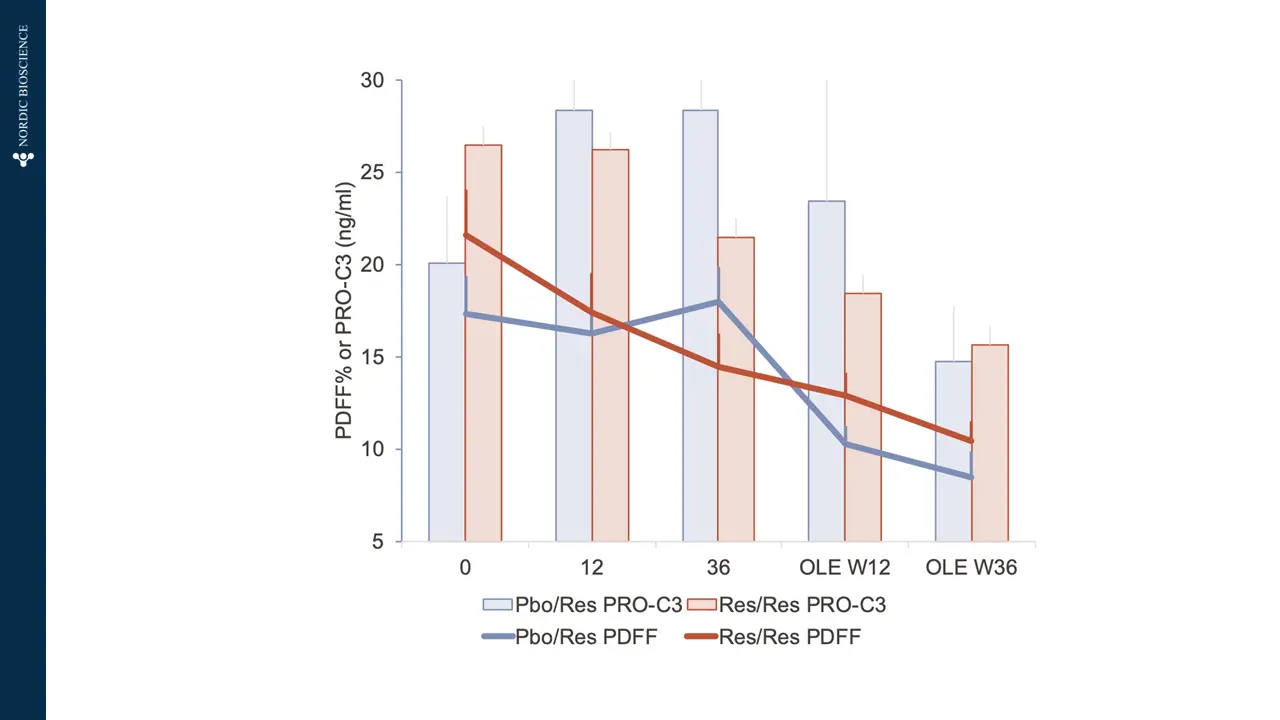
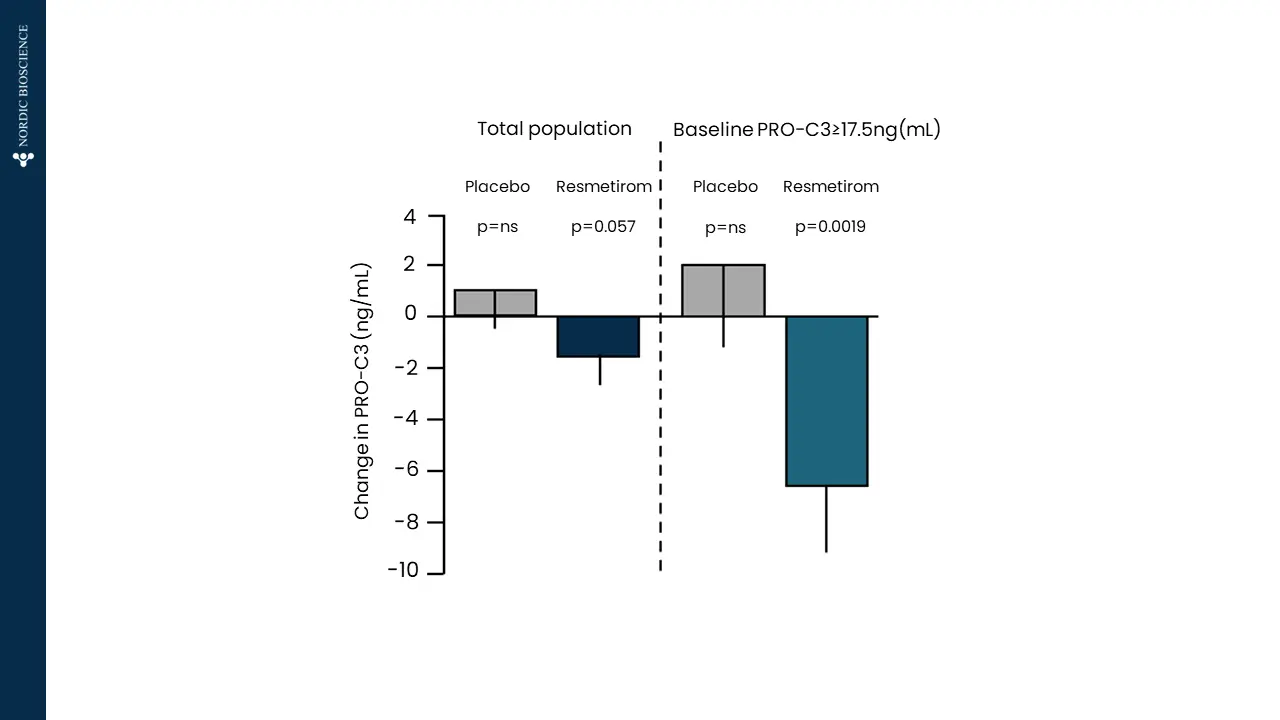
There is a pressing requirement for new, non-invasive tools to assess the severity of metabolic dysfunction-associated steatotic liver disease (MASLD), formerly known as non-alcoholic fatty liver disease (NAFLD), worldwide. These tools would aid in monitoring patient progression and stratifying risk for this increasingly prevalent condition.
Nordic Bioscience has developed biomarkers that specifically focus on the progression of liver fibrosis. These biomarkers offer a unique approach to determining the trajectory of patients with MASLD. They can be used to stratify patients based on their risk levels, assess the effectiveness of pharmacological treatments, and enhance the selection of participants for clinical trials, ultimately improving the chances of successful outcomes.