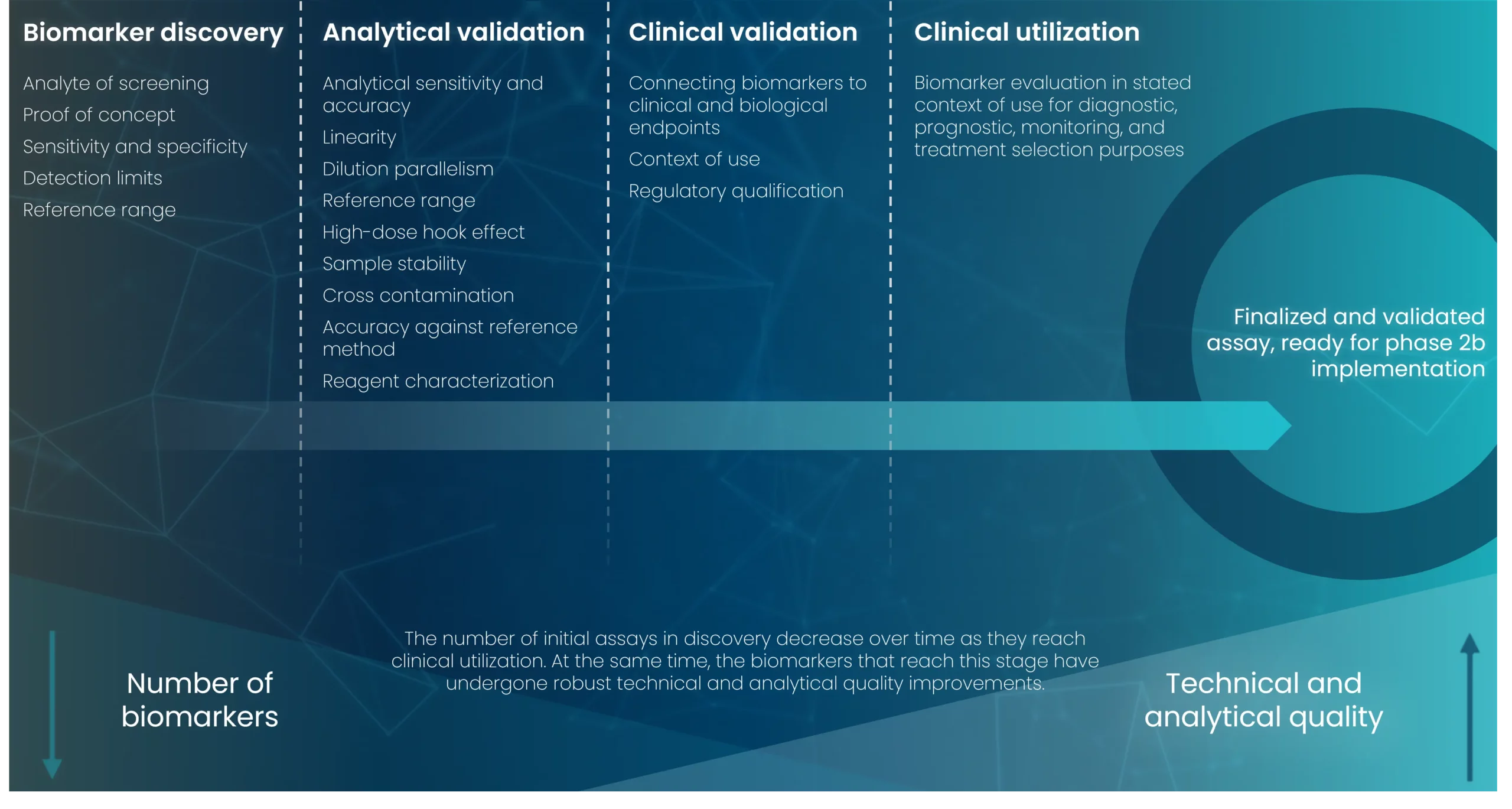
The process of biomarker qualification is a collaborative endeavor where regulatory agency personnel collaborate with the requester to guide the development of the biomarker. Due to the arduous nature of advancing a biomarker towards qualification and the requirement for robust evidence from multiple trials to support a COU, various parties often collaborate within working groups or consortia to achieve biomarker qualification.
This collaborative approach leverages shared resources, alleviates the individual burden on collaborators, and grants the group access to valuable insights that may not be accessible independently. This process was recently review by the LITMUS consortium, disclosing the full feedback from this process from both EMA and FDA, enabling other researchers to follow and use this information, to allow addition and faster Submissions.
Biomarker qualification, as mandated by the 21st Century Cures Act, follows a three-stage submission process to develop a biomarker for regulatory purposes. This process includes the submission of a LOI, a qualification plan, and a comprehensive full qualification package.
If the FDA determines the documentation submitted at each of these stages as acceptable, a communication is conveyed to the requester through a letter encompassing feedback and recommendations for further advancing the biomarker’s development. This iterative process allows requesters to collaborate with the CDER in addressing various facets of biomarker development.