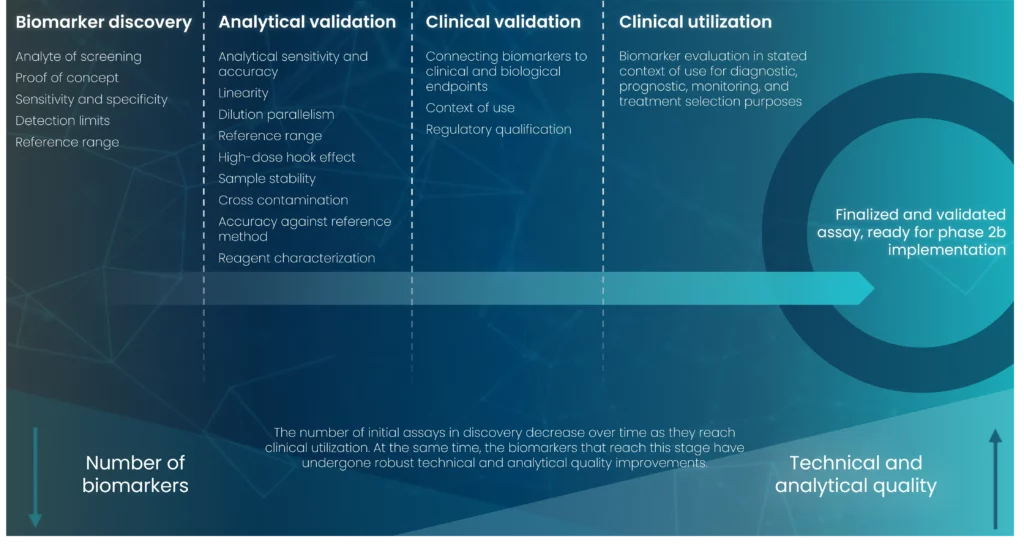
As development progresses, the clinical specificity and sensitivity of the biomarker is continuously evaluated. A great point to consider in this regard is the value of biobanking for retrospective analyses and potential discovery of novel biomarkers. During the development process, it is important to consider pre-analytical factors which can influence the process and integrity of the biomarker. These include status of the patient from which the sample is derived, biospecimen collection, handling and storage, and attention to freeze-thaw cycles of the sample.
Analytical validation is characterized by analysis of the biomarker assay performance with the goal of ensuring a repeatable measurement with low variance and good sensitivity and specificity. Multiple parameters are assessed during this phase, including selectivity, accuracy, precision, recovery, sensitivity, reproducibility, and stability. Depending on the intended use of the biomarker assay certain standards must be met, such as the Clinical Laboratory Improvement Amendments (CLIA) for assays to be used for testing human samples.
A biomarker validation according to the Clinical Laboratory and Standards Institute (CLSI) guidelines can further reduce the risk of a technical or analytical failure, thus increasing the utility of the biomarker assay, and is required for qualification and approval of the biomarker assay.