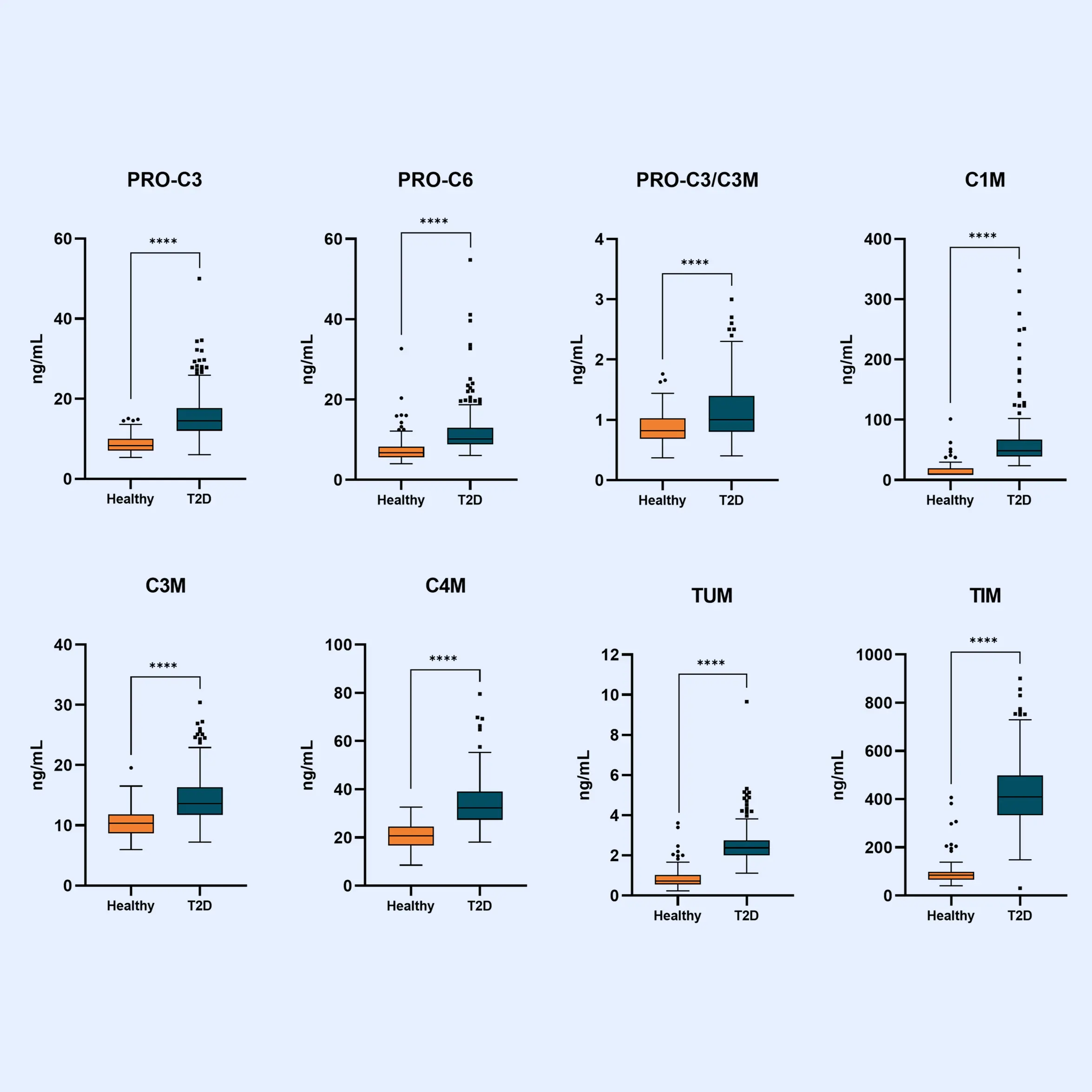
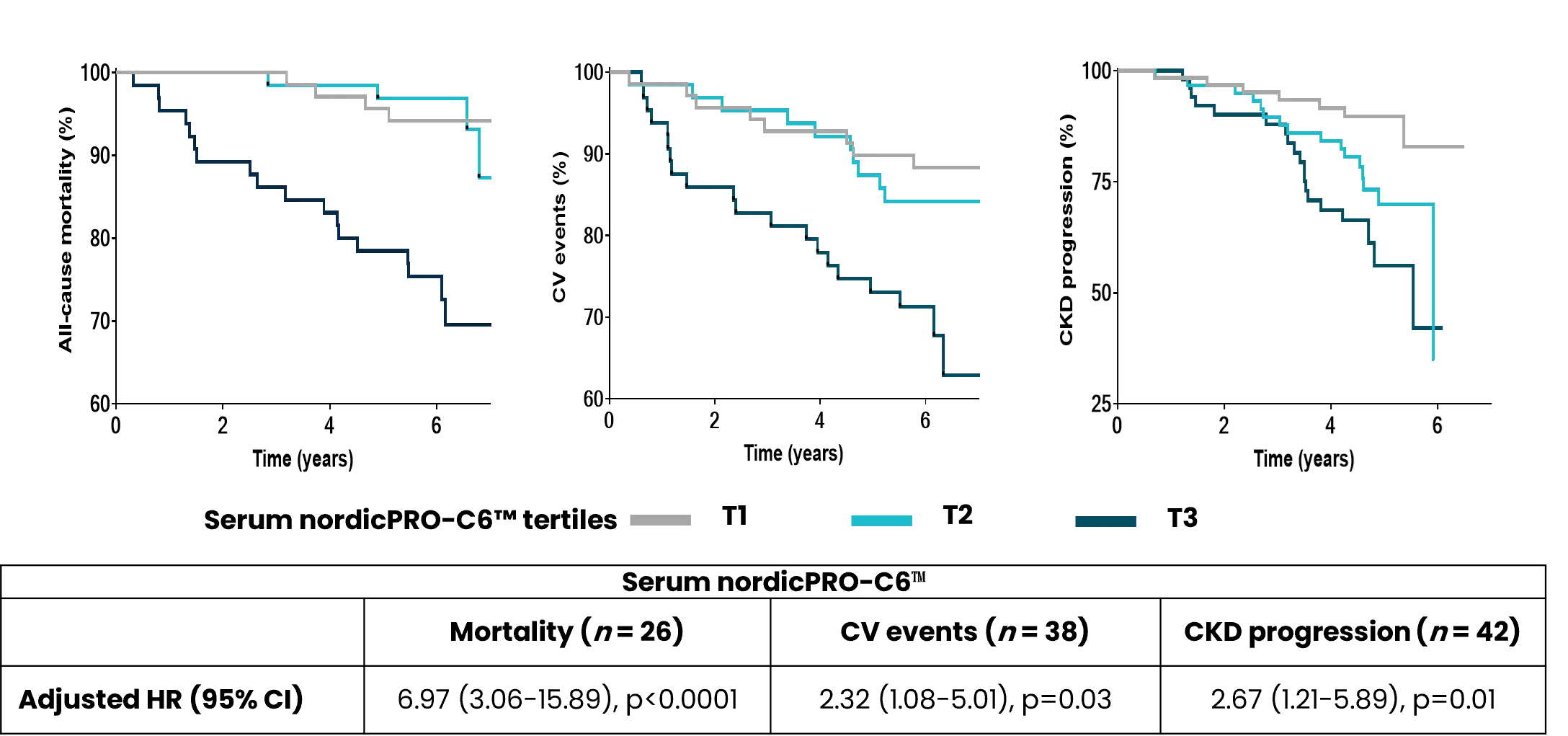
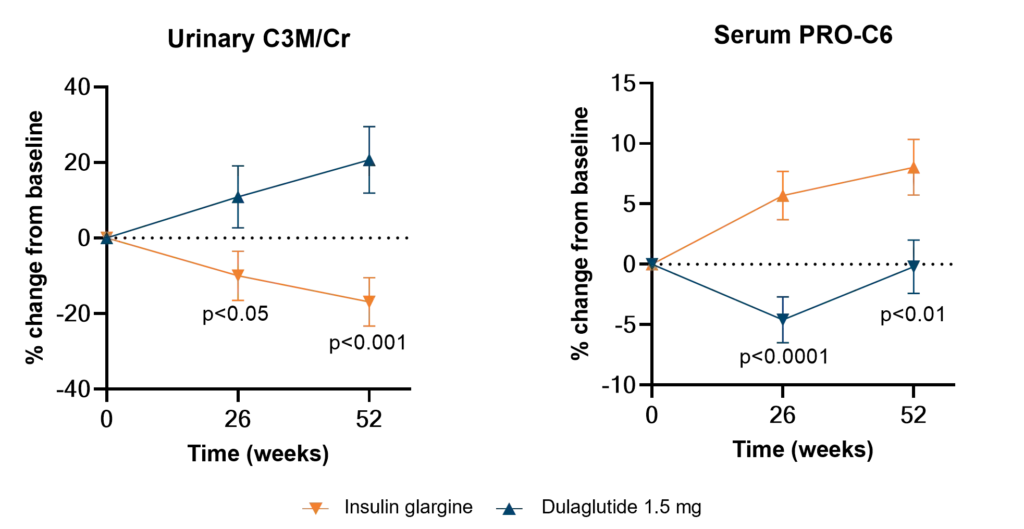
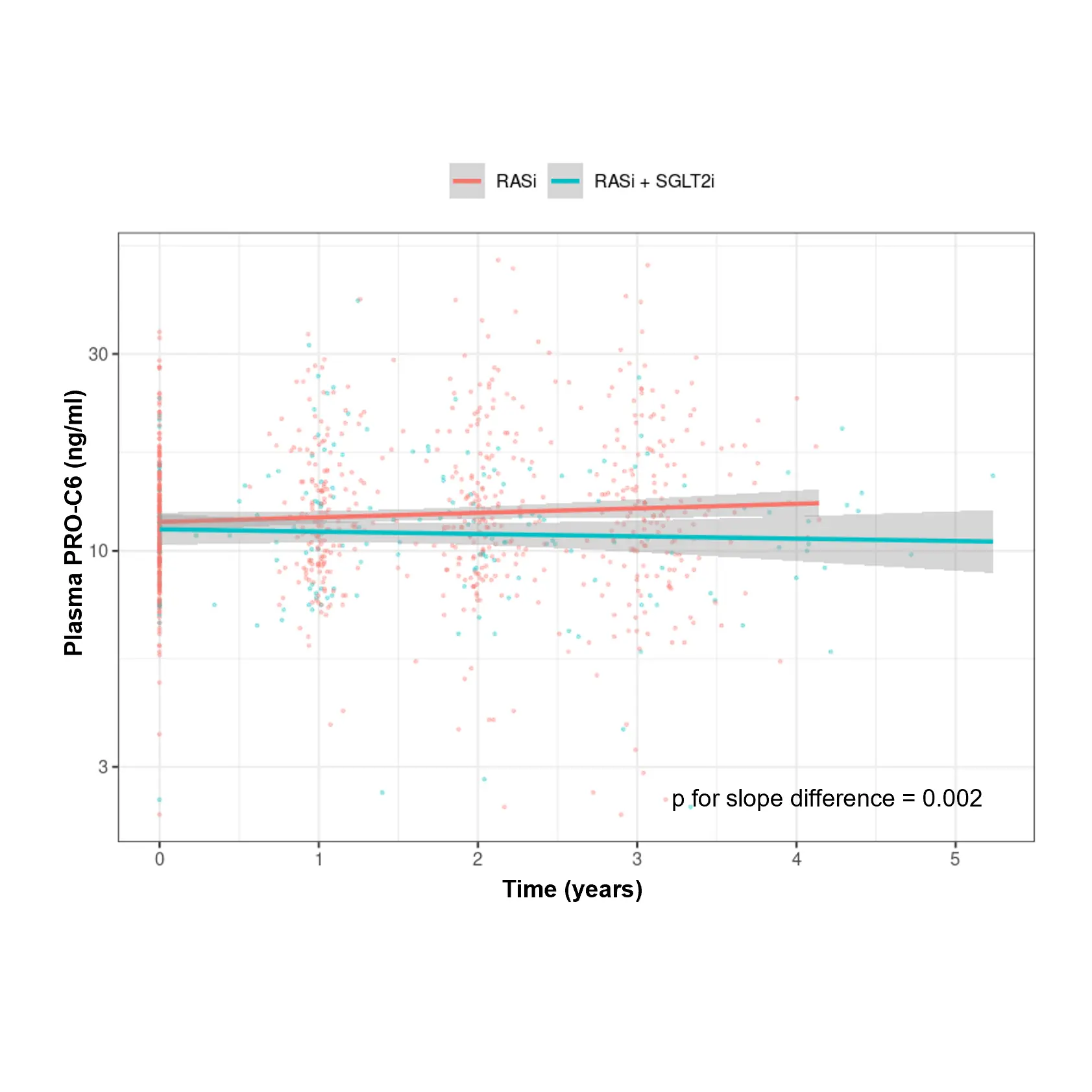
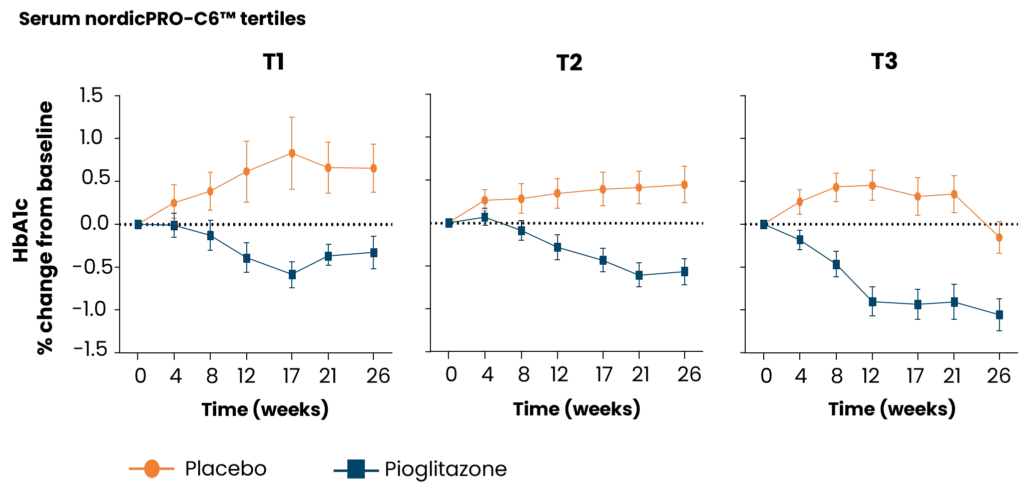
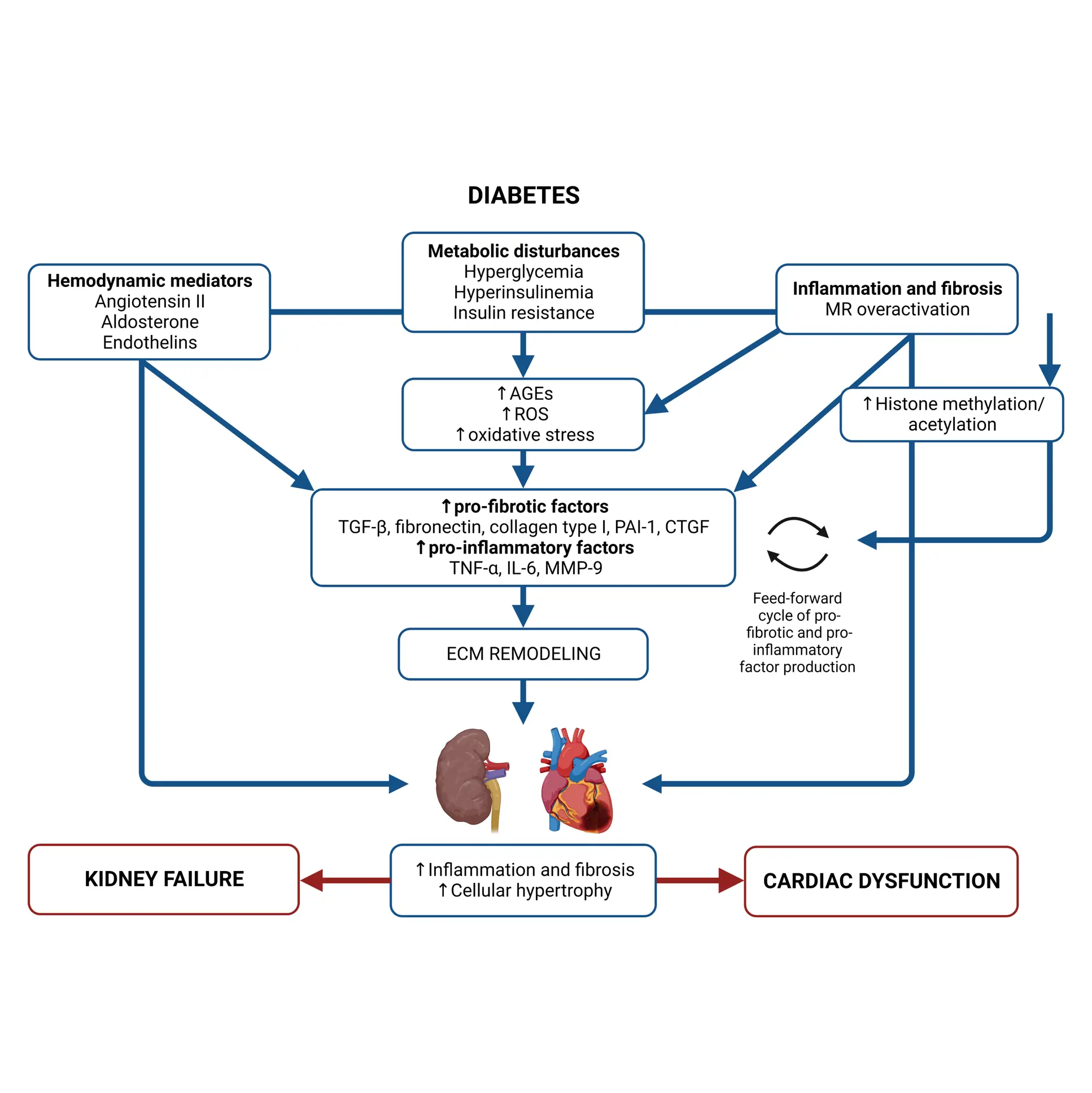
Diabetes significantly accelerates fibrotic and inflammatory processes in vital organs, yet current treatments leave a high residual risk of complications. The right biomarkers may offer a way to detect and quantify these underlying changes, particularly in kidney and cardiovascular tissues. By capturing early shifts in tissue dynamics, these biomarkers enable better risk stratification, selection, and evaluation of treatment efficacy—supporting more targeted and effective management of diabetic complications.
Despite the success of novel therapies, the residual risk of outcomes in persons with both type 1 diabetes (T1D) and type 2 diabetes (T2D) remains high. Therefore, novel biomarkers are needed to predict complications before clinical manifestations. There are currently no approved antifibrotic drugs capable of halting and/or reversing progression of kidney and/or cardiac fibrosis.
As novel antifibrotic therapies enter clinical development in early phases of chronic kidney disease (CKD), diabetic kidney disease (DKD), and cardiovascular disease (CVD), specific markers for selecting individuals and evaluating efficacy of treatment are needed.