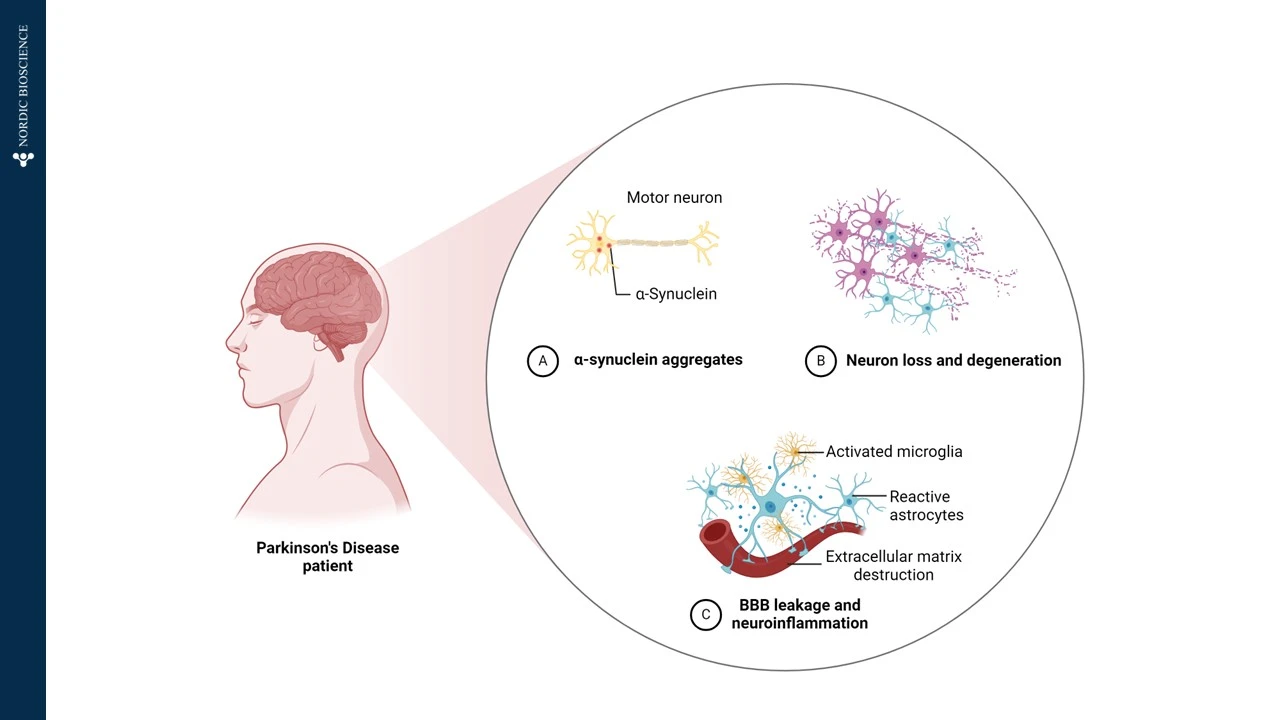
Parkinson’s Disease (PD) is a neurodegenerative disorder that primarily affecting the brain’s control over movement, thought, memory, and emotions. The disease often presents with early symptoms such as tremors, which result from impaired motor control. At the molecular level, Parkinson’s Disease is driven by a series of changes in specific proteins, particularly α-synuclein, which plays a central role in the progression of the disease.
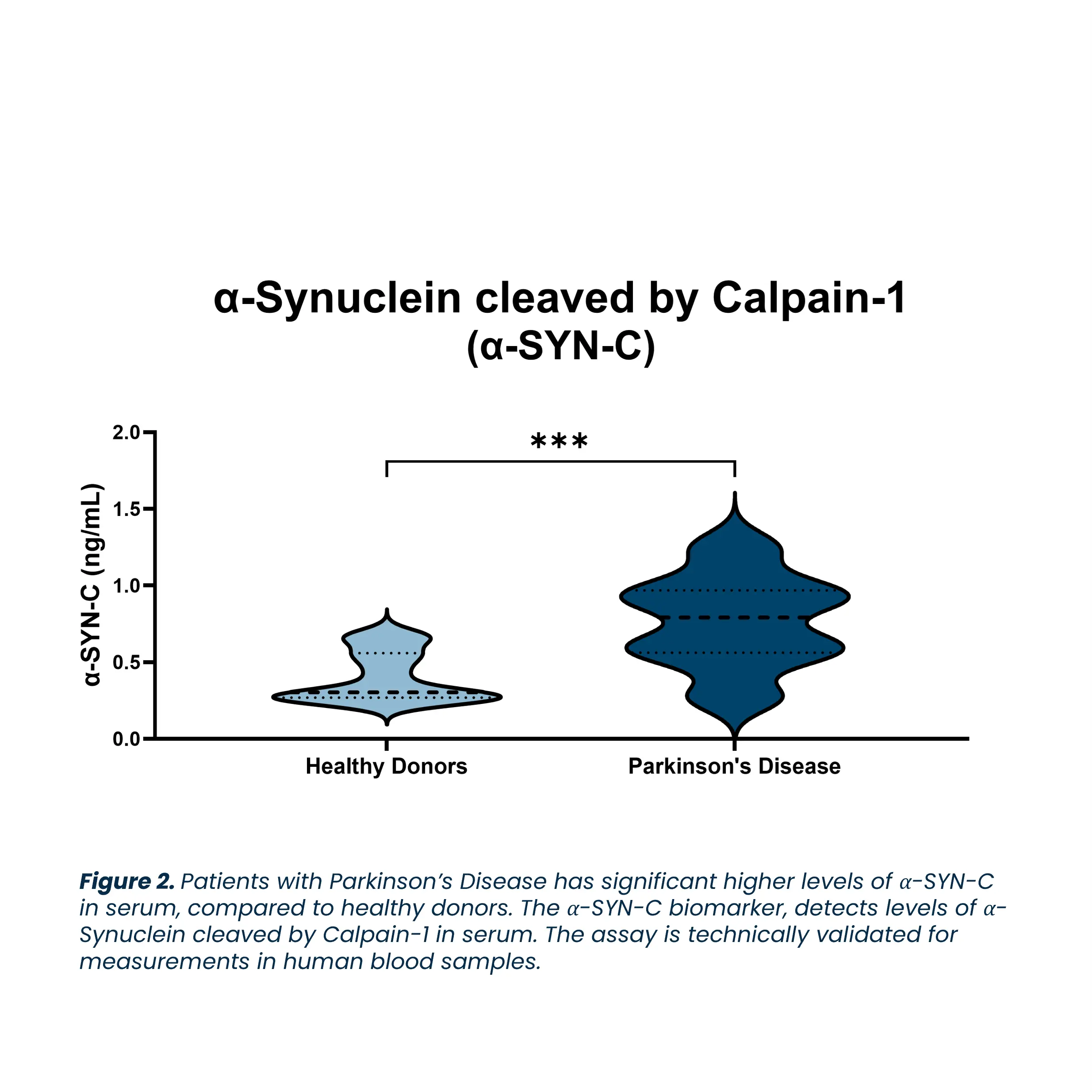
Biomarkers are essential in understanding the complexity of Parkinson’s Disease, as they help characterize the disease’s heterogeneity, allowing for better prediction and management of individual patients. The identification and tracking of biomarkers aid in diagnosing PD, predicting prognosis, assessing treatment responses, and identifying new therapeutic targets. This comprehensive approach helps address the unmet needs of Parkinson’s Disease, providing crucial insights into disease progression and potentially improving treatment strategies for patients.