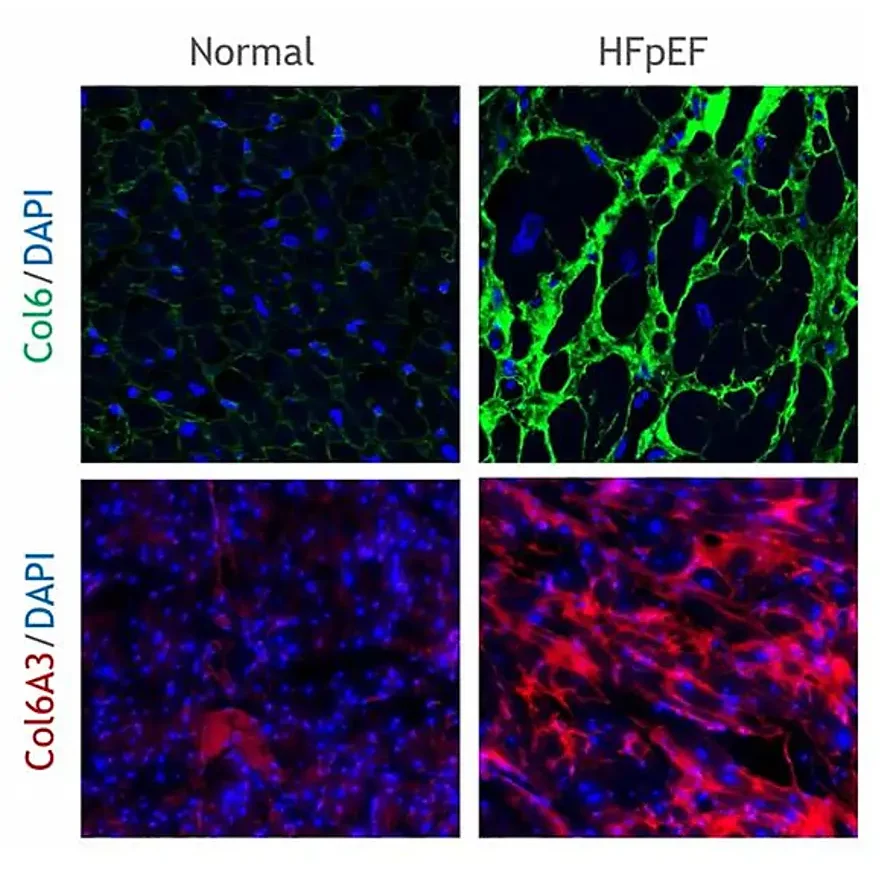
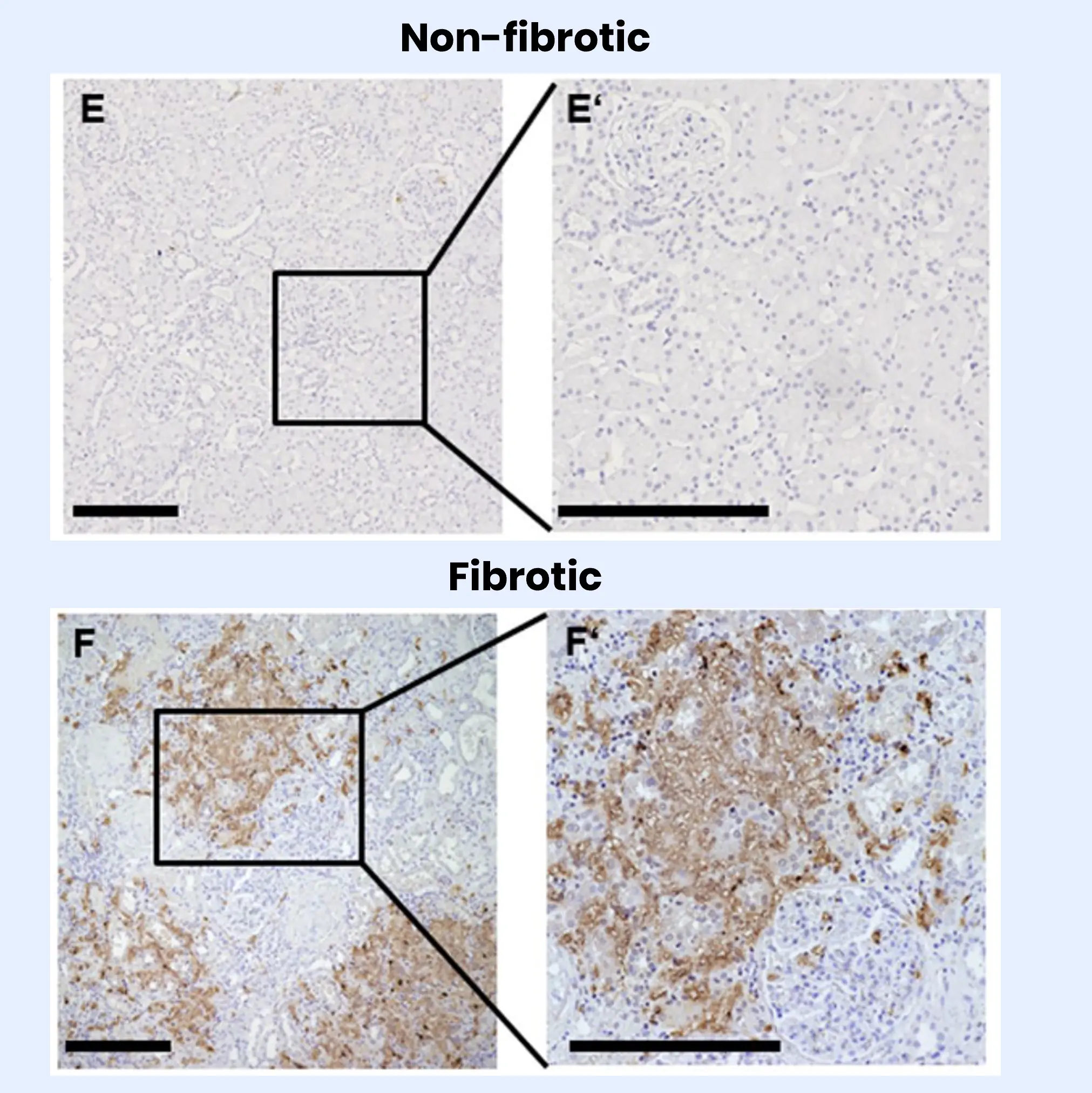
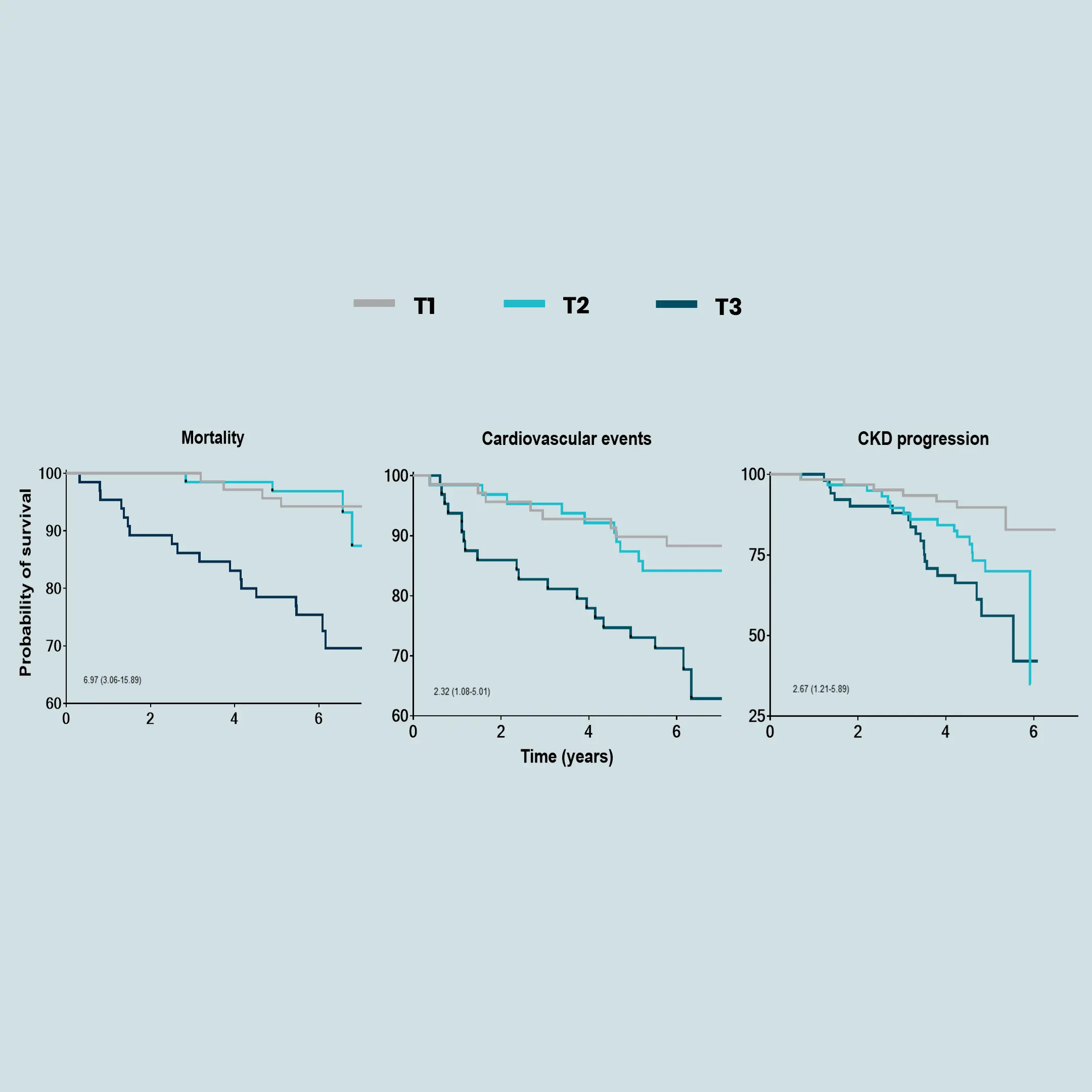
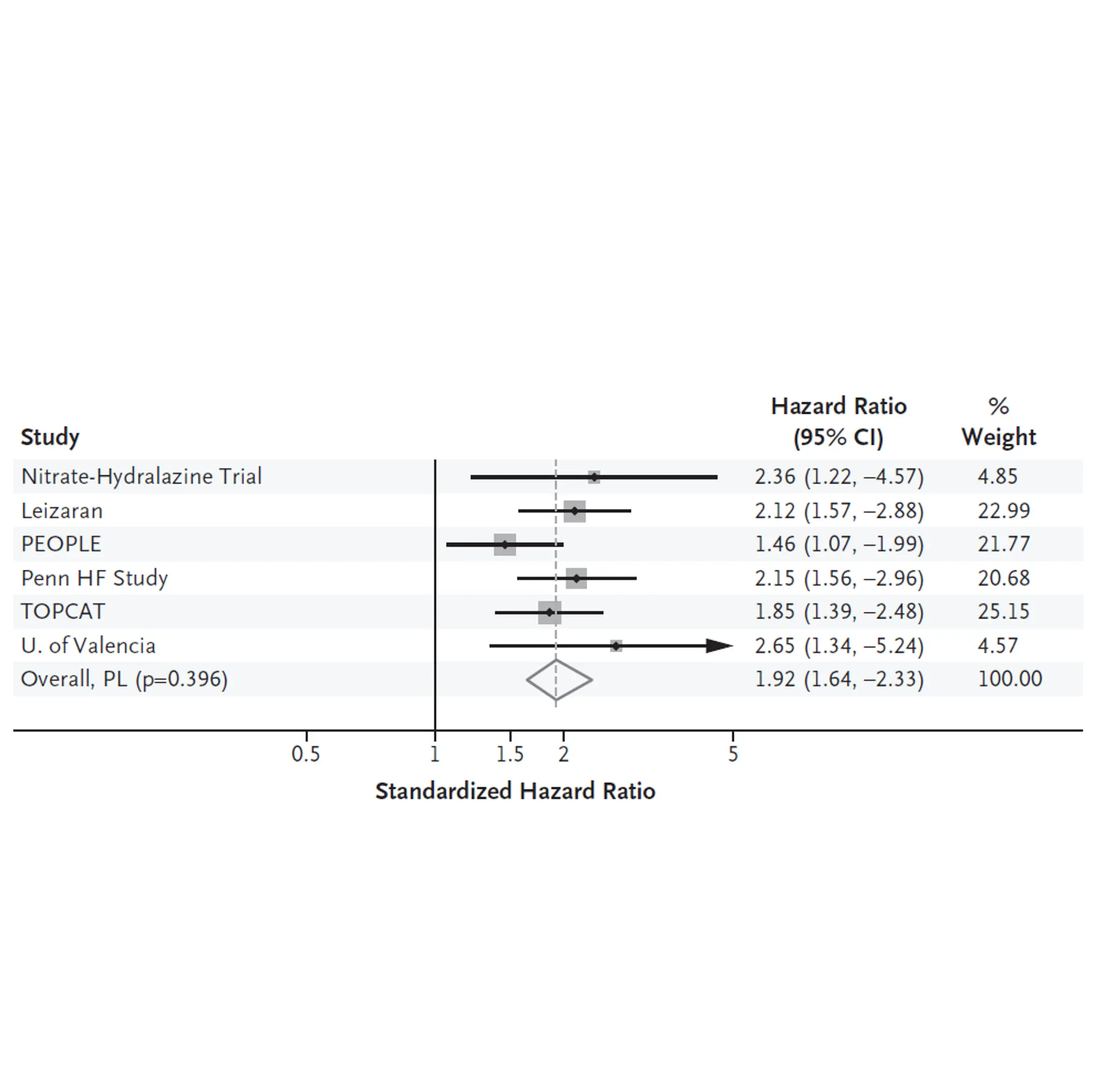
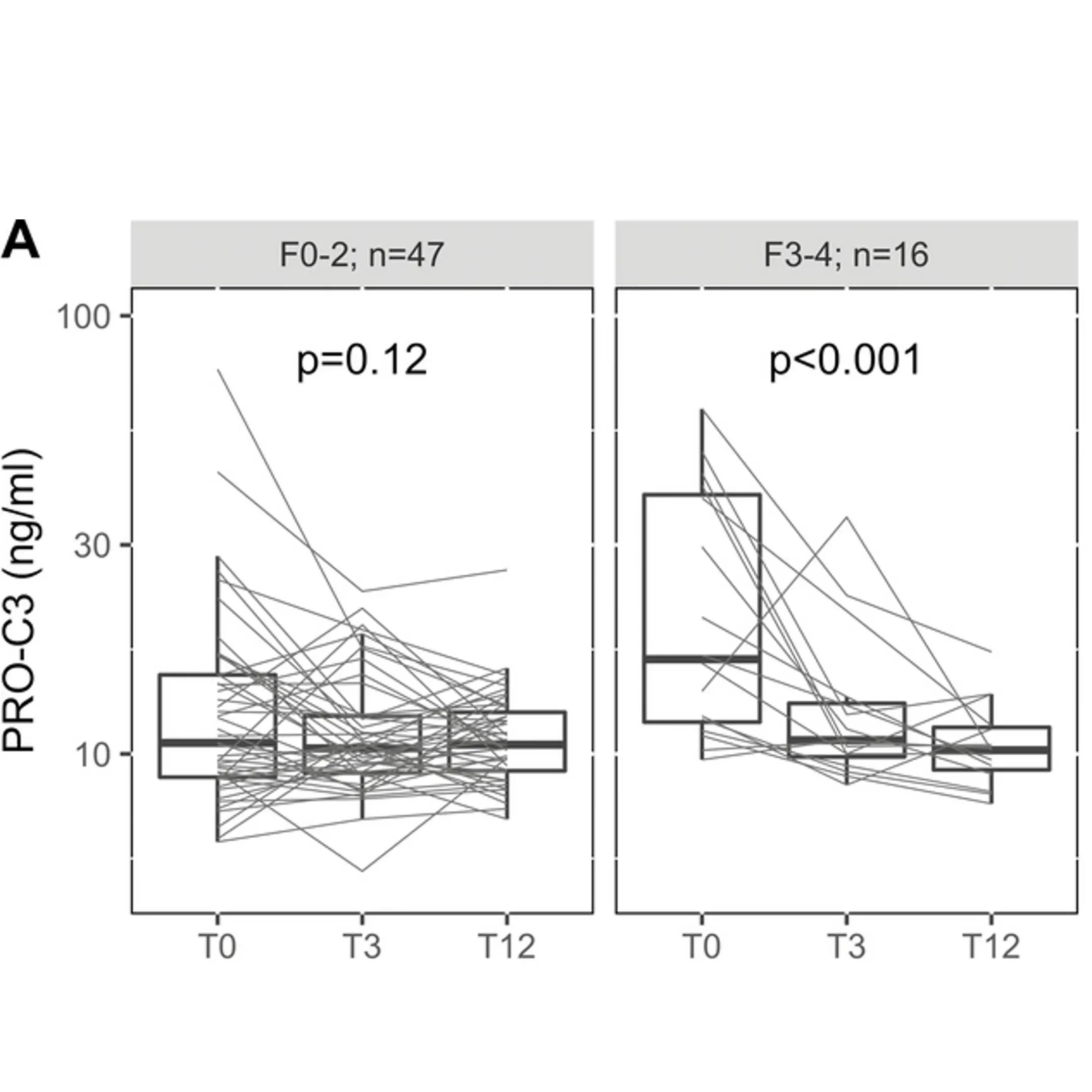
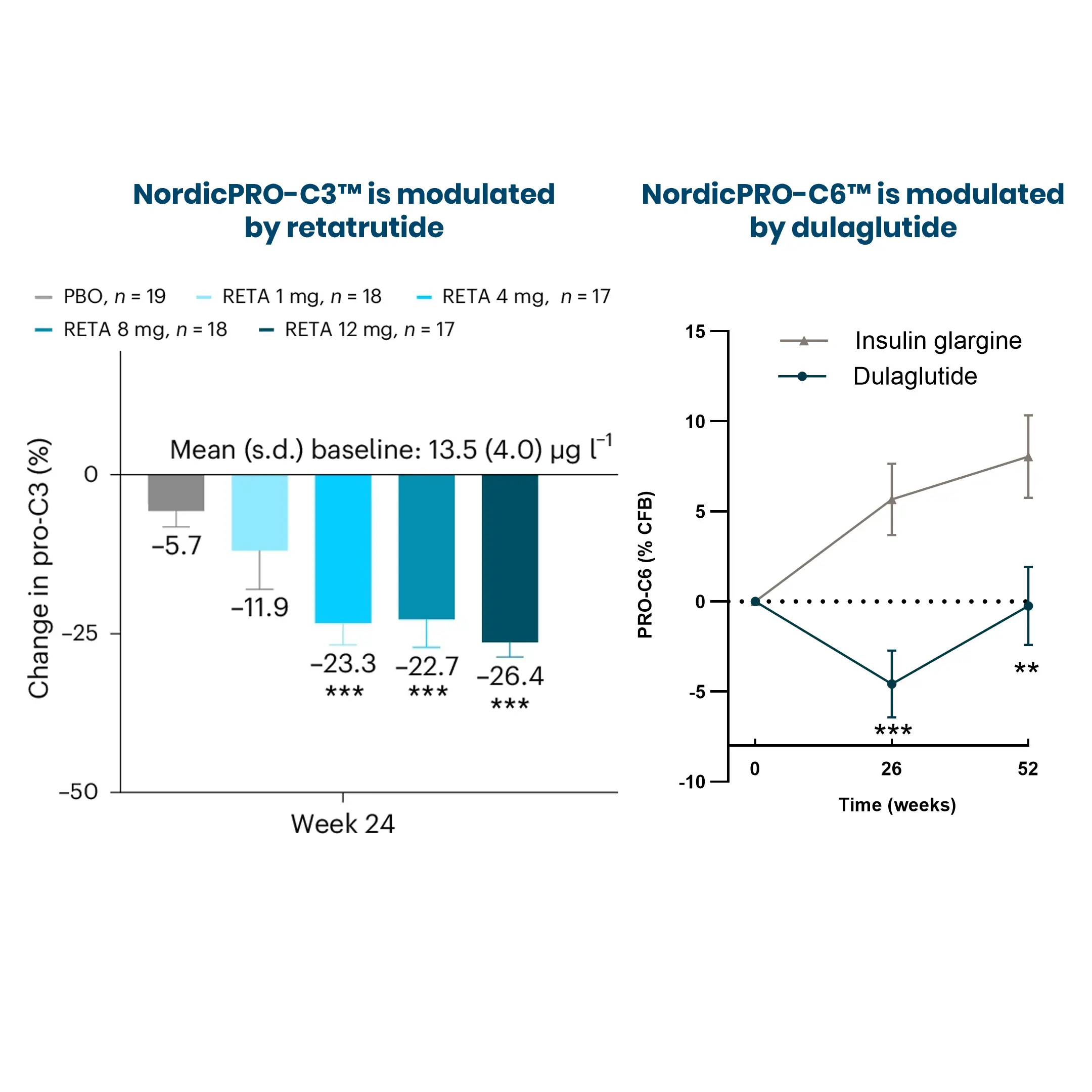
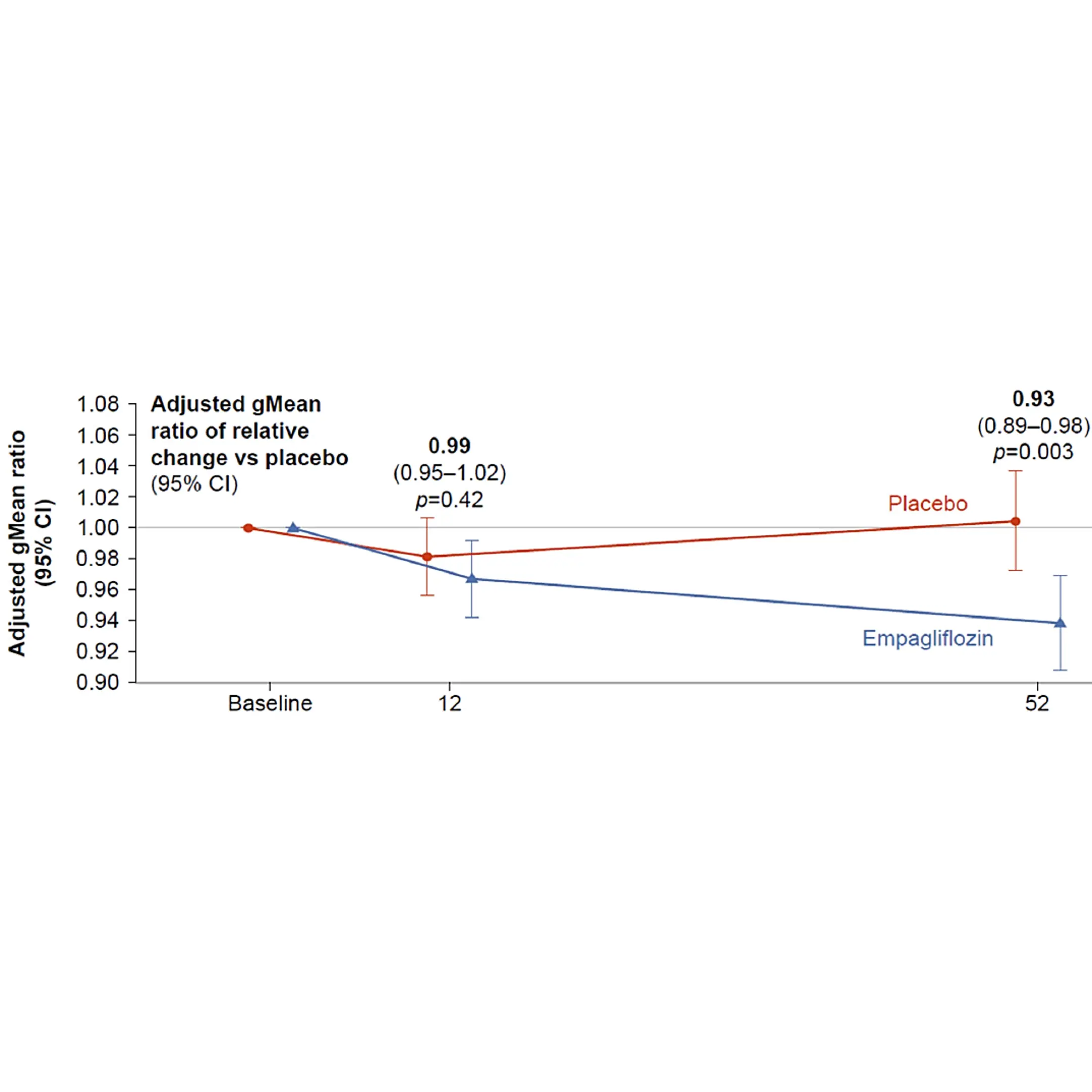
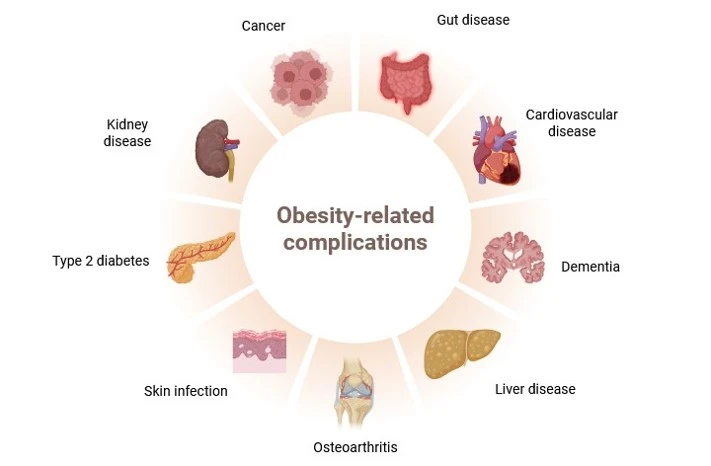
Obesity is a major risk factor for metabolic dysfunction-associated steatohepatitis (MASH). It is linked to diastolic dysfunction and heart failure with preserved ejection fraction (HFpEF), and is an independent risk factor for chronic kidney disease (CKD). Therefore, there is a current need for novel biomarkers to support personalized care for people with obesity.
BMI alone does not reflect metabolic health. Biomarkers can help with diagnosis and stratification by identifying pre-obesity states or people at high risk before severe weight gain. Biomarkers can guide personalized treatment and identify who will respond to specific interventions, such as lifestyle changes or glucagon-like peptide-1 receptors agonists (GLP-1 RAs). Lastly, biomarkers can help monitor treatment response and predict long-term outcomes, such as cardiovascular disease (CVD).
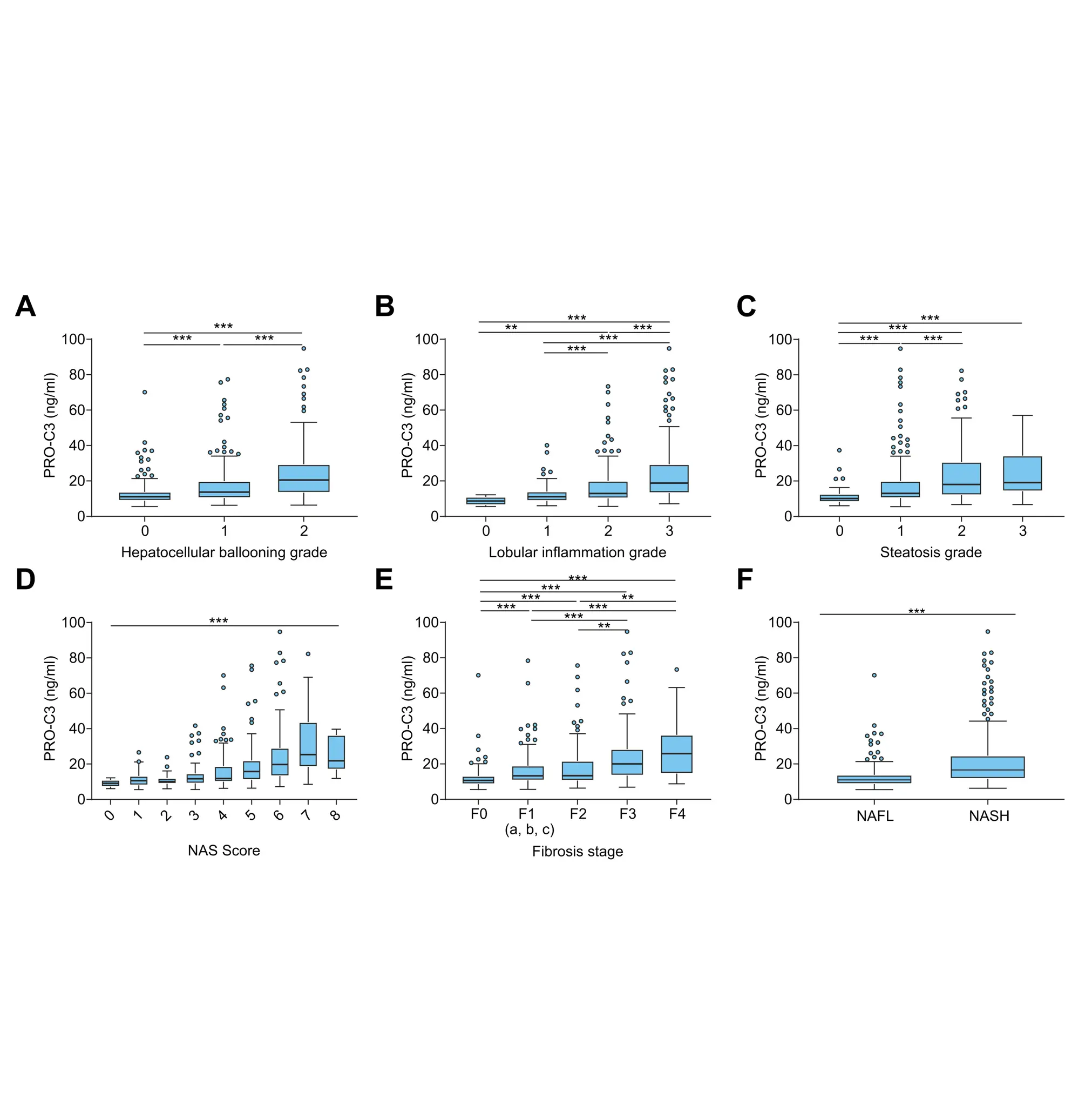
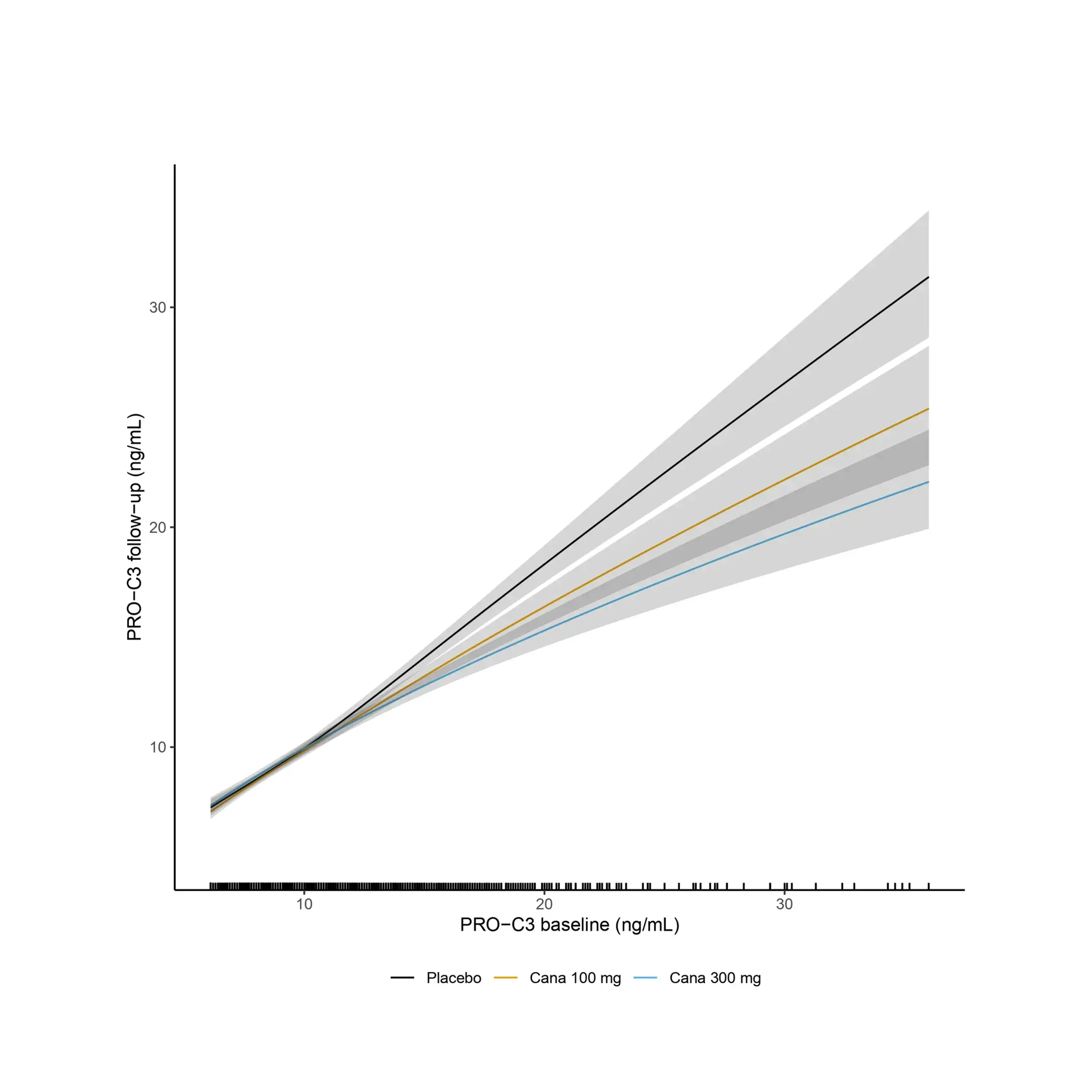
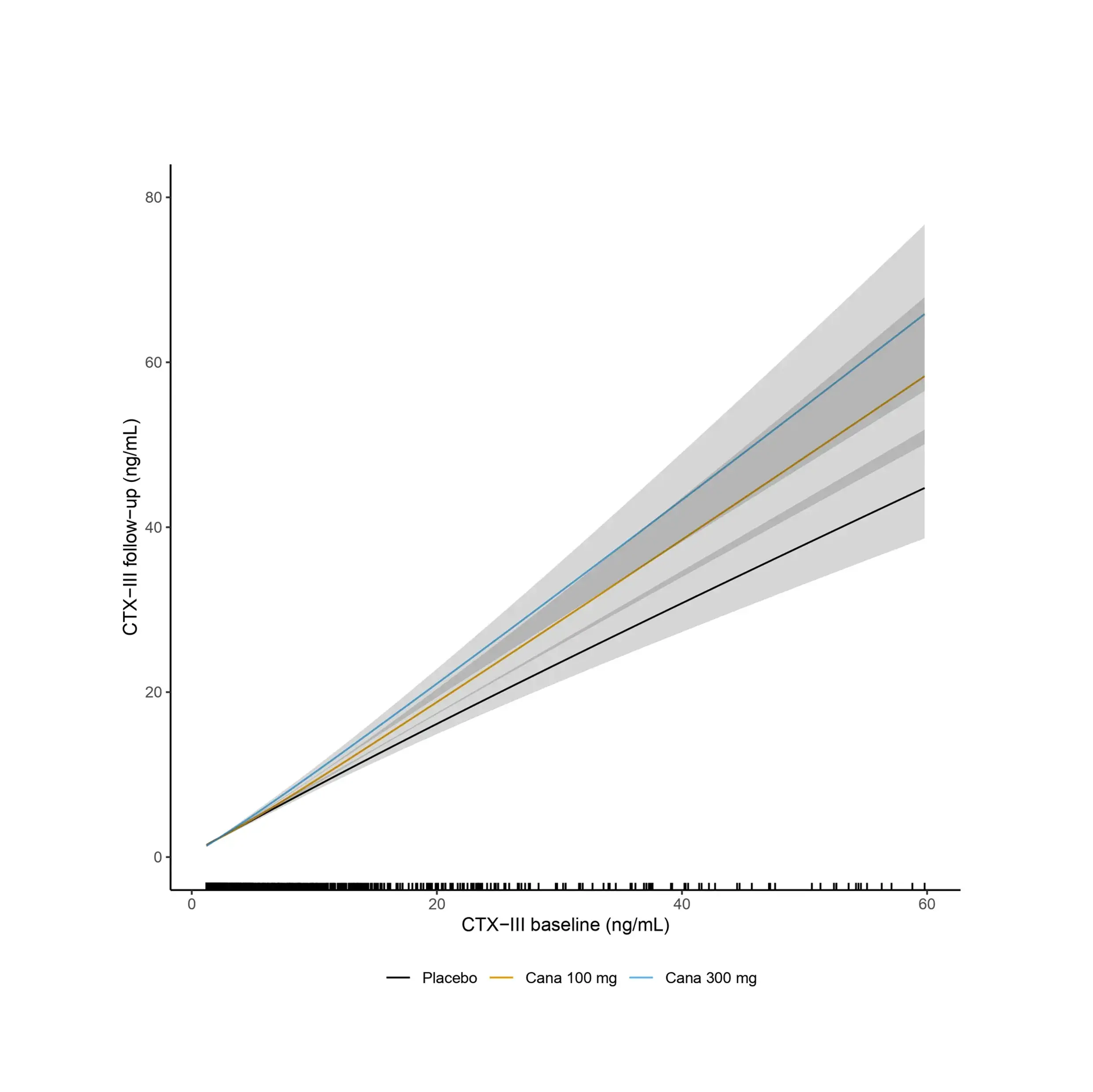
Increased fibroblast activity in people with obesity promotes excessive extracellular matrix (ECM) production, leading to tissue fibrosis and contributing to the progression of organ dysfunction and related diseases.
Nordic Bioscience’s biomarkers, which reflect ECM turnover, can be used to study and improve our understanding of fibrotic processes in organ systems affected by obesity.