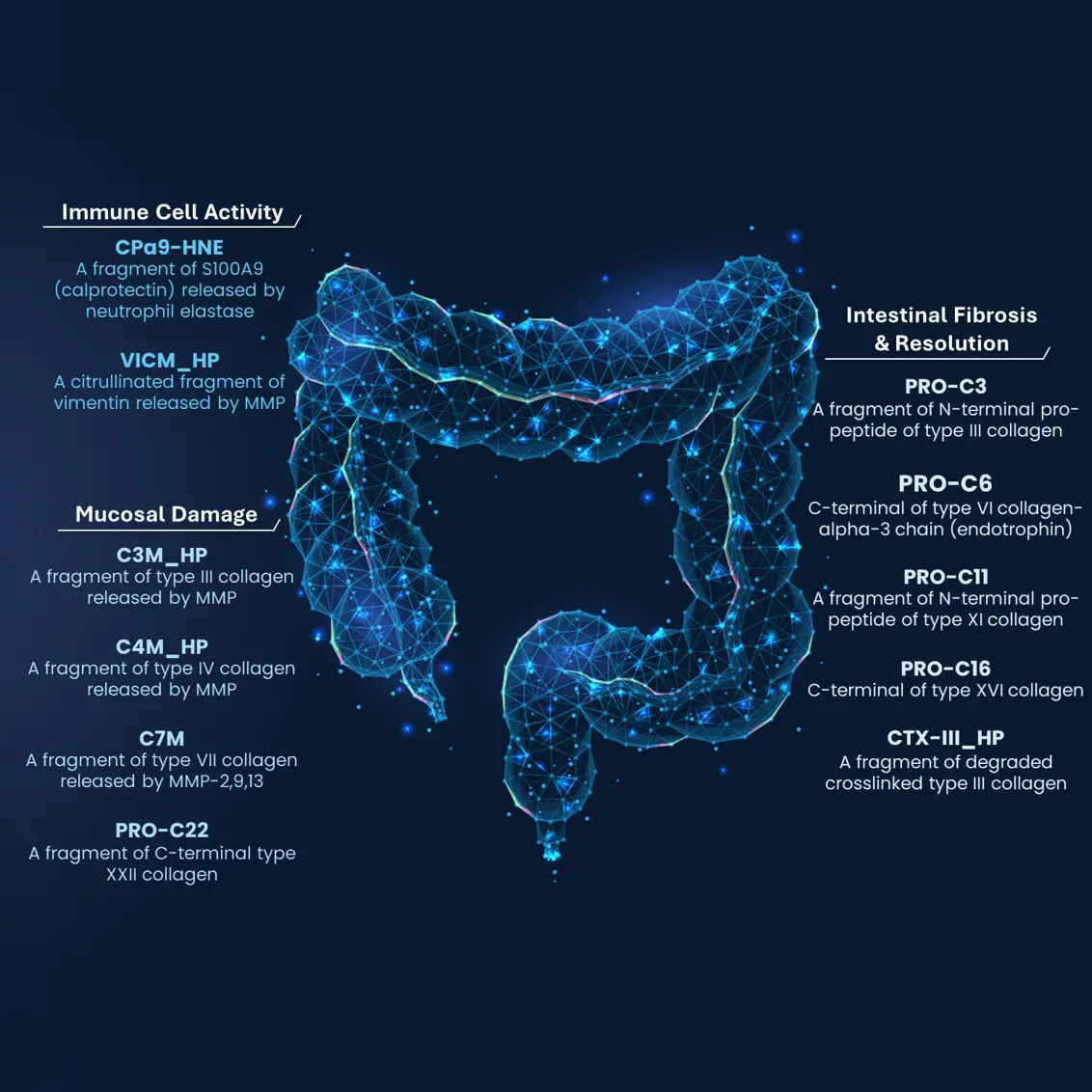
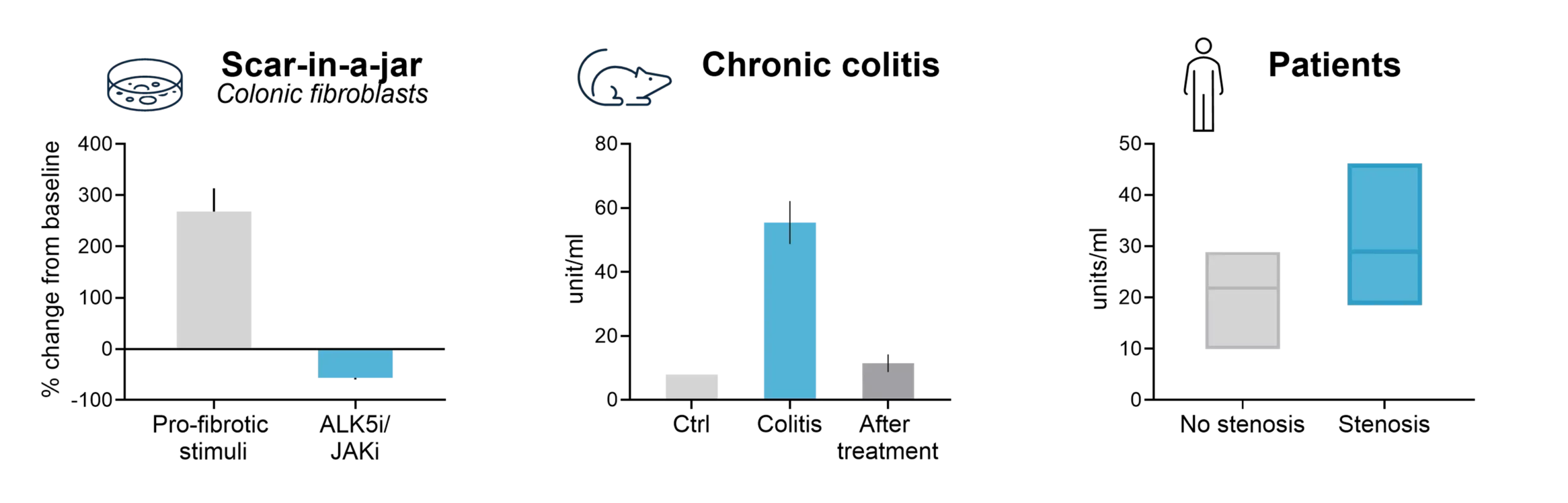
During chronic inflammation, immune cells secrete pro-inflammatory cytokines that activate tissue-resident fibroblasts, the primary producers of ECM proteins such as collagen. This fibro-inflammatory process leads to progressive tissue stiffening and, ultimately, stricture formation.
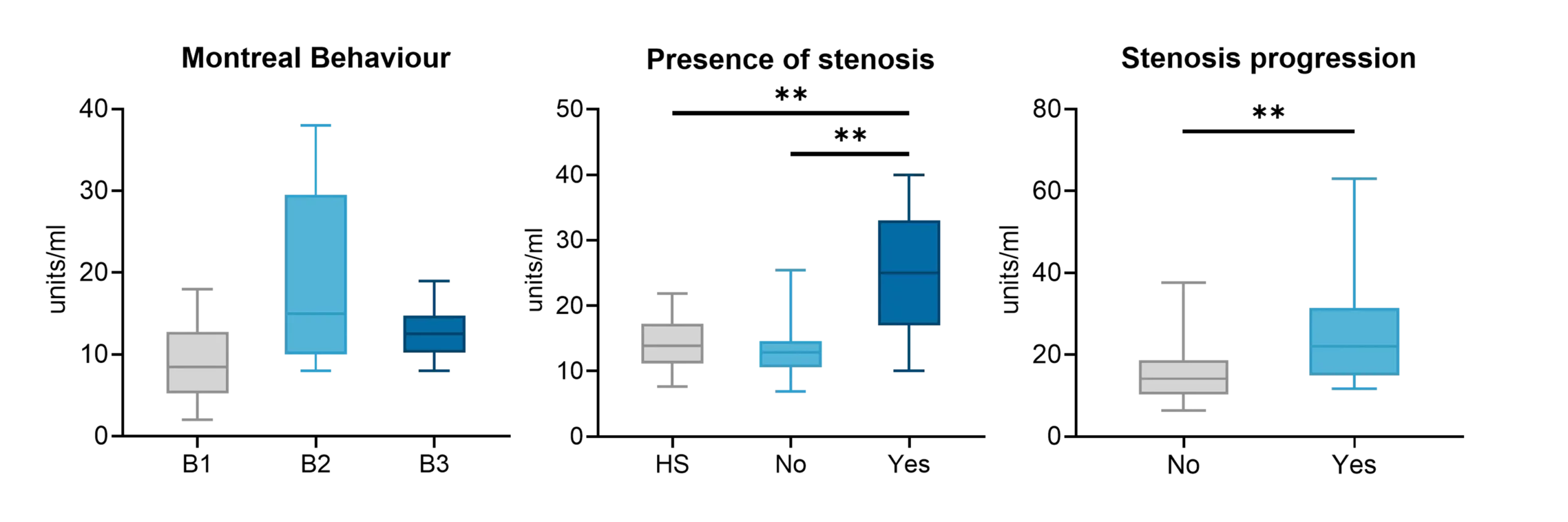
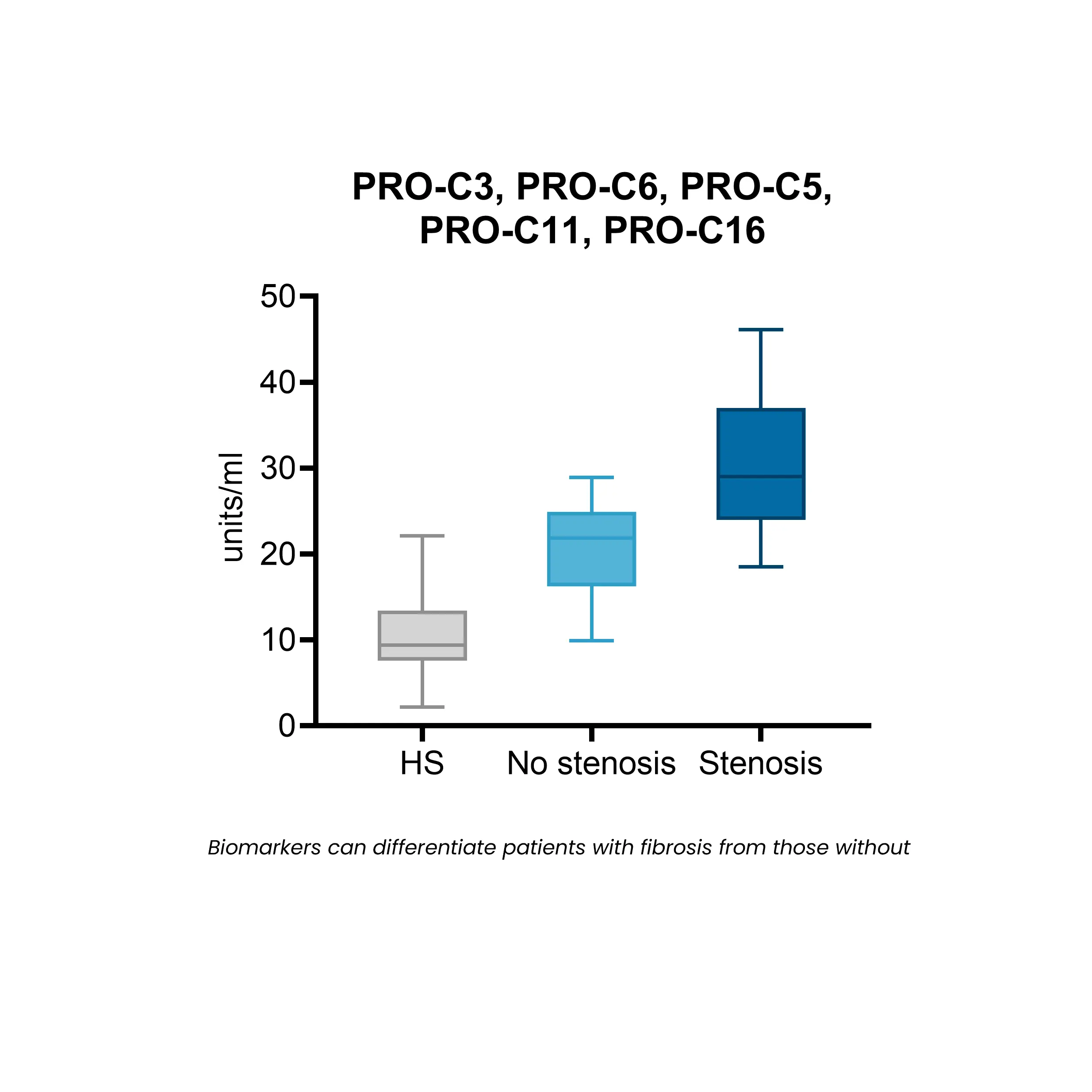
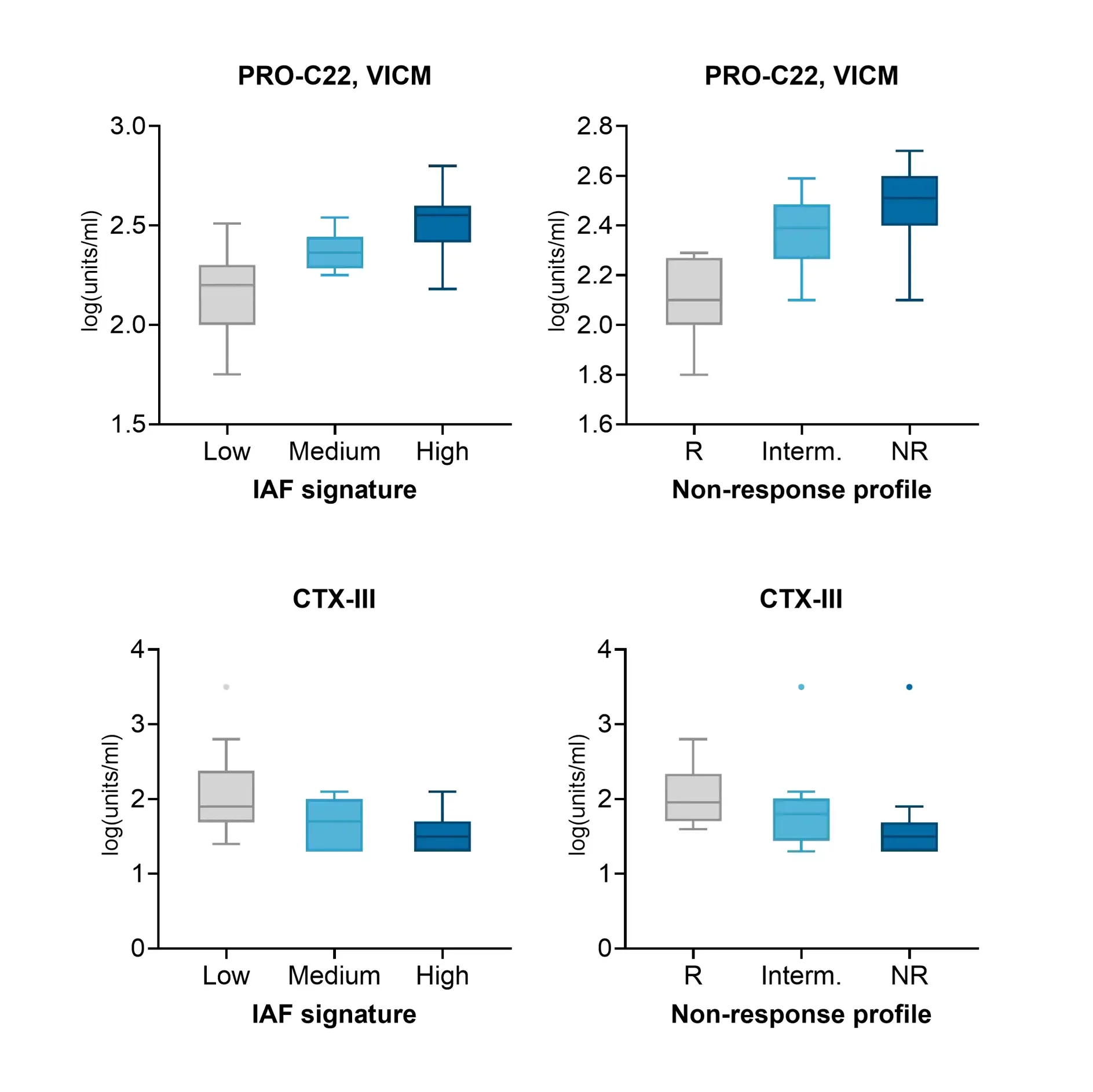
Fibrostenotic Crohn’s disease has been associated with non-response to therapeutics, with approximately 50% of patients developing fibrotic complications during their disease course.
In ulcerative colitis, fibrosis is increasingly being recognized as a clinically relevant complication that impacts long-term disease management.
The NordicIBDTrace™ biomarker panel offers a means to quantify intestinal fibro-inflammation, providing valuable insights that can support the development of antifibrotic therapies aimed at preventing or treating fibrostenotic complications.