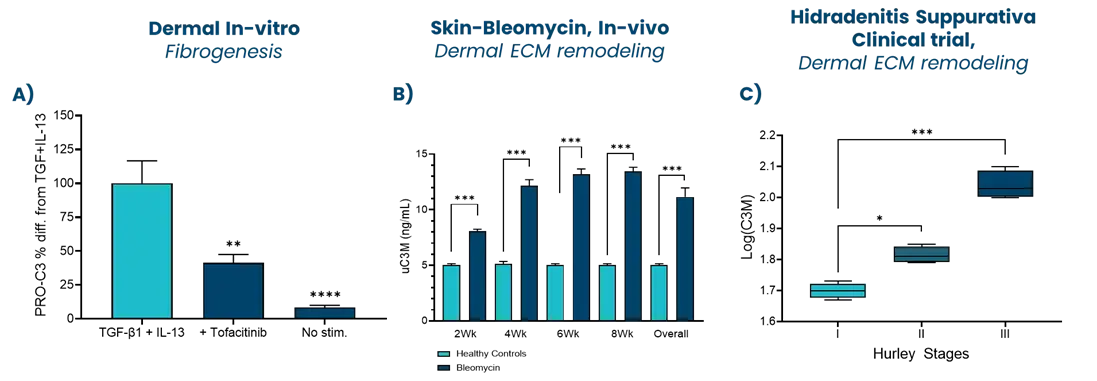
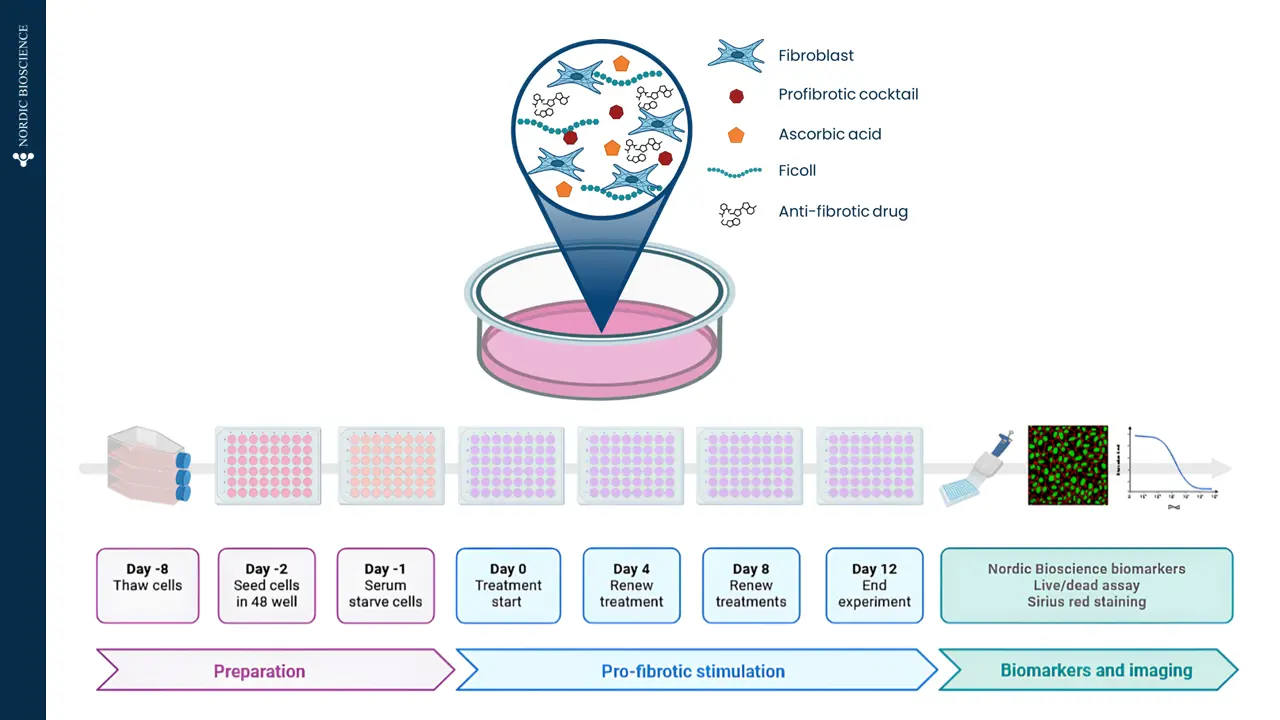
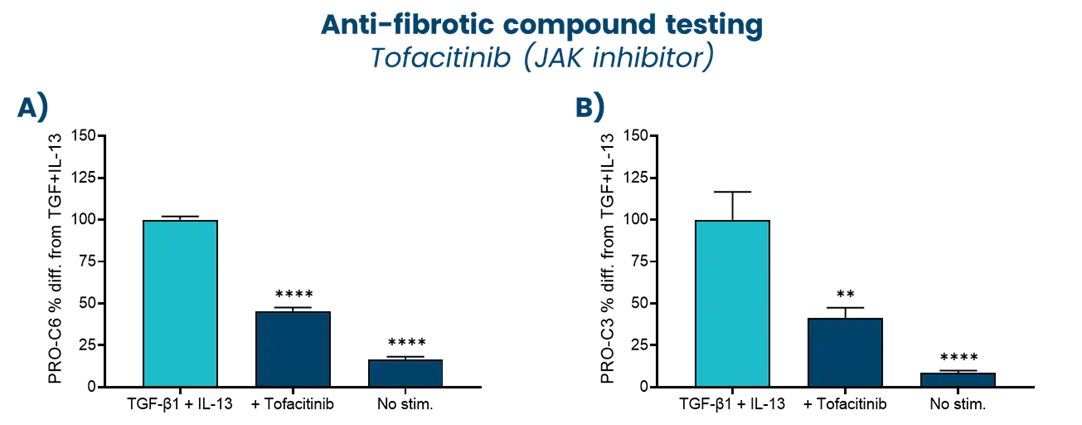
A drug development route for success is key but often challenging and full of uncertainties. Strategies that can increase the likelihood of success are vital. The FDA and EMA recognize that biomarker-based drug development strategies could be the solution in combination with translation research. This approach offers a biology-focused pathway that reduces time and cost and enhances the overall likelihood of success.
Nordic Bioscience combines innovative translational models with the Nordic ProteinFingerPrint Technology™ to translate findings effectively. This is facilitated by utilizing the same biomarkers across different clinical stages of development creating a link between the preclinical model and the clinic. This strategically selected development route greatly enhances the efficiency of drug development pipelines.
In essence, the integration of biomarker-based drug development strategies in translational research not only accelerates the progression of therapeutics but also increases the likelihood of success. Through advanced translational models in combination with the ProteinFingerPrint™ biomarkers, Nordic Bioscience contributes significantly to the optimization of decision-making processes in clinical development, ultimately ensuring better treatments for the patients.