Nordic Bioscience Introduces the FIB-NIT™ Biomarker Panel for Fibrotic Diseases
May 3, 2024
A valuable tool for accelerating clinical trials in fibrotic diseases and reducing financial burden
FIB-NIT™ (Fibrosis Non-Invasive Tests) is an innovative panel that utilizes biomarkers of fibrotic processes. These biomarkers have been proven to associate with superior pharmacodynamic and prognostic performance to investigate dynamics within fibrotic diseases, including understanding drug efficacy in such indications —demonstrated in over 100 publications in relevant and peer-reviewed scientific journals.
Fibrosis is a pathological condition characterized by the disruption of the balance between formation and degradation of extracellular matrix components (ECM), as well as exacerbated fibroblast activity and immunological modulations leading to excessive accumulation of collagens resulting in tissue scarring and organ dysfunction. Collagens are major components of ECM that are affected by fibrosis in the injured tissue, which produce unique fragments. These unique fragments, called neo-epitopes, are released in the bloodstream where they can be non-invasively measured by the Nordic ProteinFingerPrint Biomarker Technology™ to understand ECM turnover disruption. The FIB-NIT™ panel consists of the fibrogenesis NordicPRO-C3™, the fibrolysis NordicCTX-III™, and the outcome risk NordicPRO-C6™ biomarkers.
NordicPRO-C3™
Function: PRO-C3 measures the formation of type III collagen, a key process behind fibrogenesis development and progression across different organs and tissues (liver, lung, kidney, heart, intestines, skin). PRO-C3 assesses the exact epitope released by activated fibroblasts during type III collagen formation.
Relevance: PRO-3 correlates with fibrosis severity. PRO-C3 concentrations dynamically change with disease severity and provide an estimation of the level of active fibrogenesis This allows:
- Monitoring anti-fibrotic treatment response and pharmacodynamics;
- Identifying patients who are likely to respond to treatment;
- Superior performance;
- Stratification of patients based on disease severity;
- Identifying patients with advanced fibrosis;
- Stratify patients according to the risk of developing clinical outcomes.
When combined with other clinical variables in the ADAPT score, PRO-C3 outperforms other liver scores widely used in the clinics for identifying advanced liver fibrosis.
NordicPRO-C6™ (also known as Endotrophin)
Function: PRO-C6 measures the formation of type VI collagen which is connected to fibrogenesis and activated fibroblasts. This marker assesses Endotrophin, a hormone responsible forpromoting profibro-inflammatory processes via signaling mechanisms.
Relevance: PRO-C6 acts as a driver of fibro-inflammatory processes, providing an efficient tool in predicting outcomes (risk of cardiovascular events; mortality; disease progression) in many different fibrotic diseases affecting liver, heart, kidneys, gastrointestinal system. Endotrophin also has a major role in the development and progression of metabolic disorders and is highly correlated to angiogenesis.This allows for identifying patients suffering fromheart failure with preserved ejection fraction (HFpEF), and patients at risk of developing cardiovascular events and those who are at risk of death.
NordicCTX-III™
Function: CTX-III measures the proteolytic degradation of cross-linked type III , a process behind fibrolysis and fibrosis resolution.
Relevance: CTX-III correlates with fibrosis resolution and improvement of fibrosis following interventions (drug treatment and lifestyle change). CTX-III also identifies patients with endotypes who have progressive, stable or regressive fibrosis profile. Together with PRO-C3, CTX-III can measure the balance between fibrolysis and fibrogenesis.
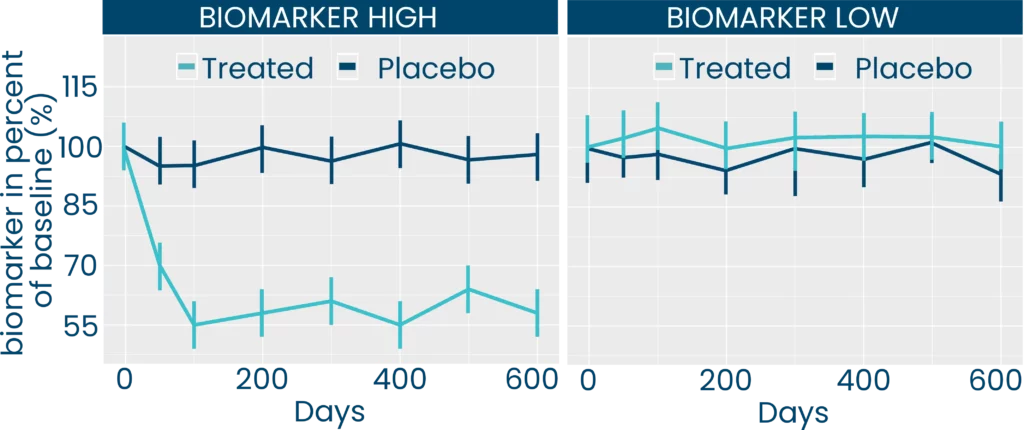
When combined in the FIB-NIT™ panel, these three biomarkers offer a valuable tool for accelerating clinical trials and reducing financial burden. FIB-NIT™ achieves this by helping with patient selection and inclusion, identification of treatment responders, assessment and prediction of drug response without the need for invasive procedures like biopsy; or the use of expensive noninvasive screening tests that are commonly used in the clinical practice.




