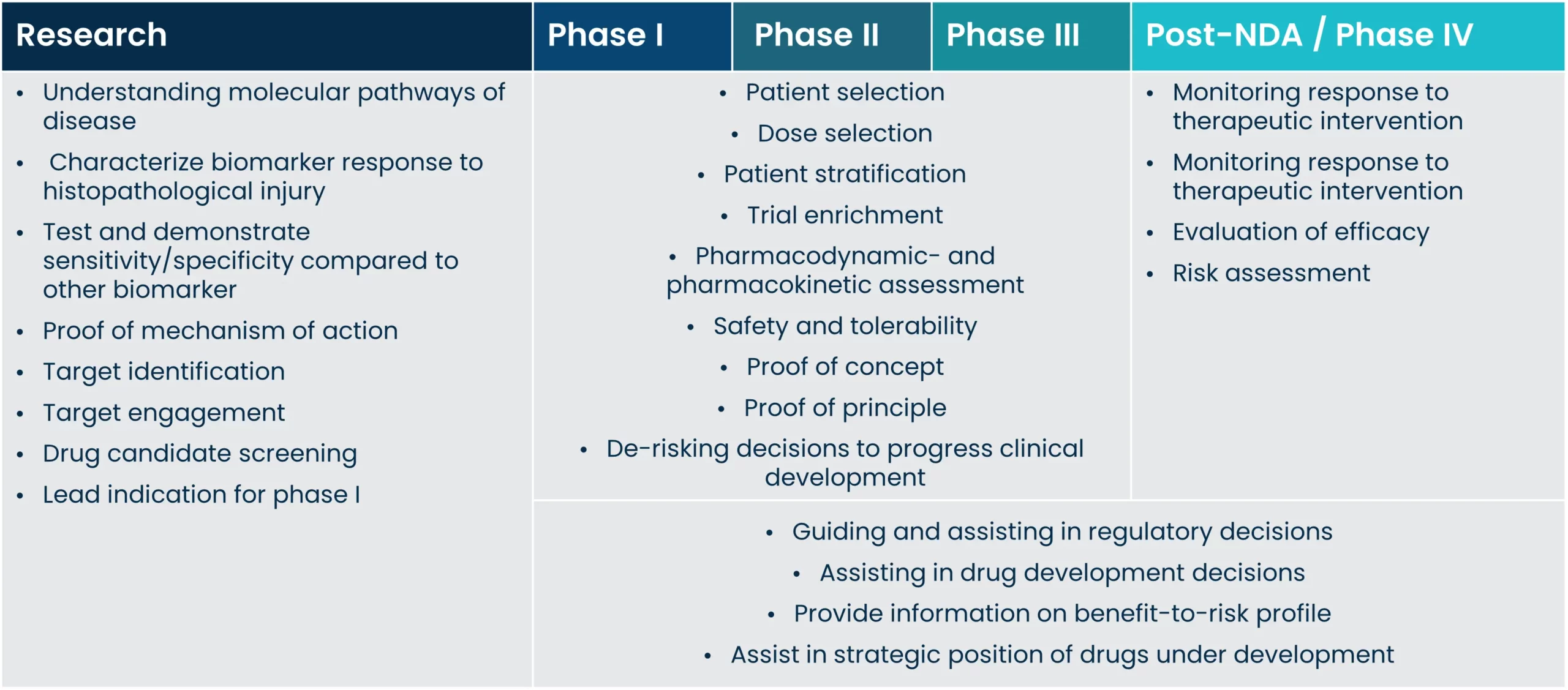
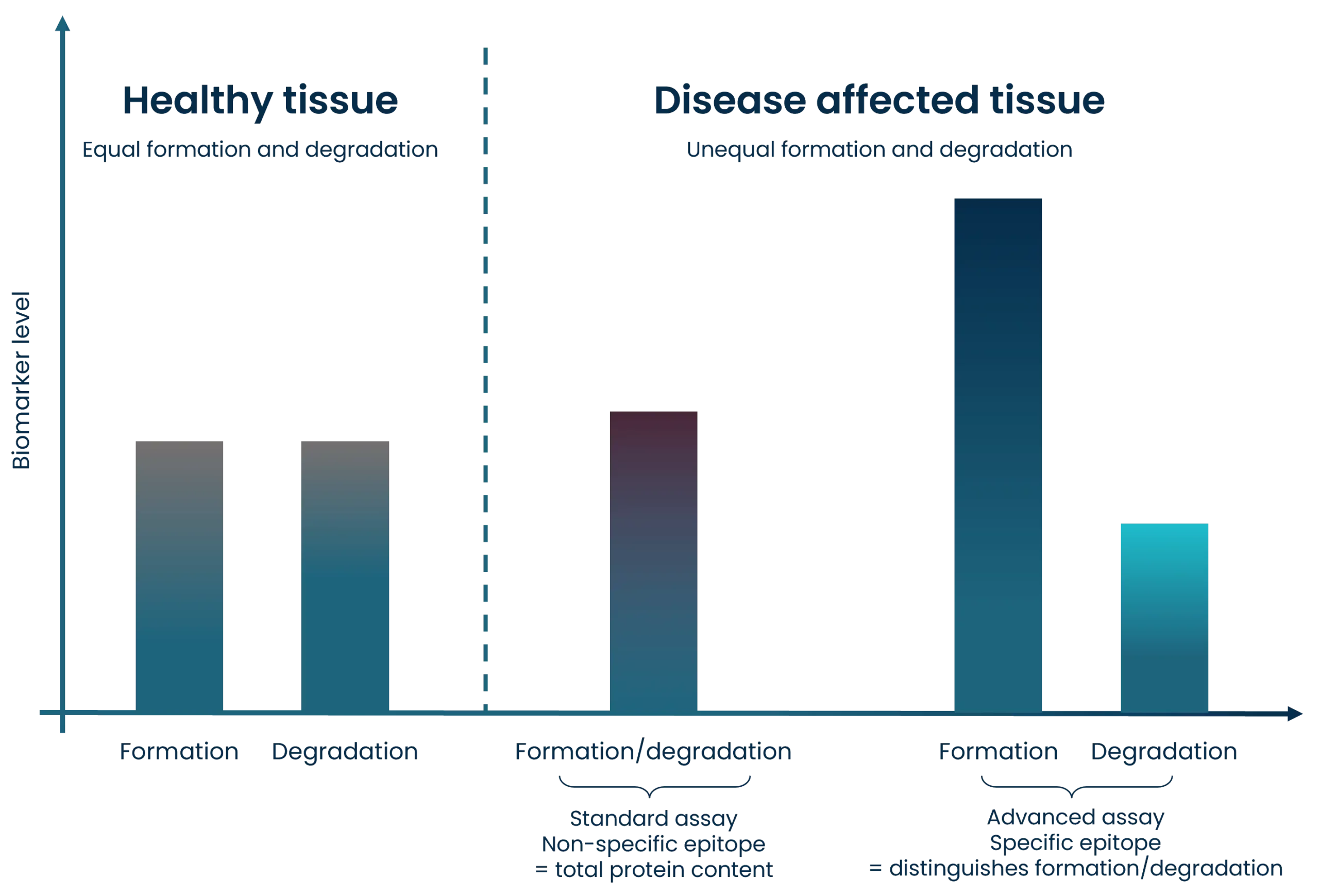
A biomarker is a characteristic of a biological process that occurs naturally or during pathogenesis, and it can serve as a function of therapeutic intervention. Biomarkers are an essential part of drug discovery, all the way from pre-clinical discovery to clinical validation and implementation, and the importance of biomarkers in decision-making continues to increase.
Better informative biomarkers can increase the likelihood of drug advancement or approval, and implementing biomarkers increases the success rate in drug development.
Clinical decision making is relying more and more on the importance of biomarkers. However, biomarkers validated to high standards, and biomarkers that reflect biological and pathological processes accurately are still a growing area. Such biomarkers are needed to develop treatments faster, and to improve and guide clinical trial design by selecting and de-selecting patients.