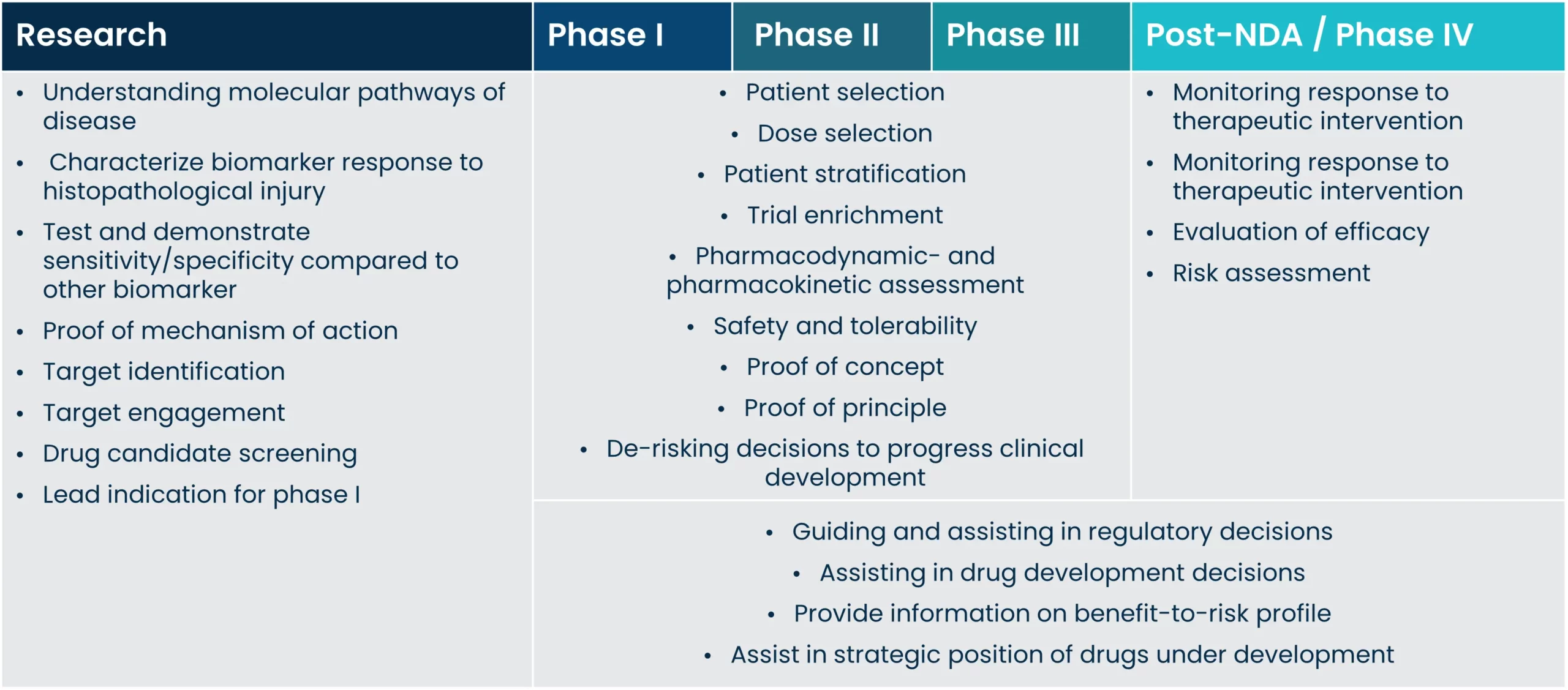
Implementing biomarkers on all levels of drug development has been proven to facilitate better and faster drug development, and improve the outcomes of clinical trials. Biomarkers are important for all stakeholders involved in drug development; patients, payers, regulators, and drug developers all alike, as the right biomarkers can lead to cost-effective trial design.
This is achieved by reducing trial duration before reaching significant endpoints, by preventing unnecessary treatment, or by reducing testing and evaluation of patients. Improving these factors could also lead to improved patient-reported outcomes through an overall improvement in quality of life.
The clinical utility of a biomarker is first and foremost confounded in its ability to accurately diagnose the presence of disease and provide information on the course of disease and subsequent risk of outcome. Once such relationships are established, the biomarker can be used for several different clinical applications, such as pharmacodynamic studies, trial enrichment and monitoring of disease development and treatment response. In direct association with these uses there is also the potential of assisting treatment decisions.