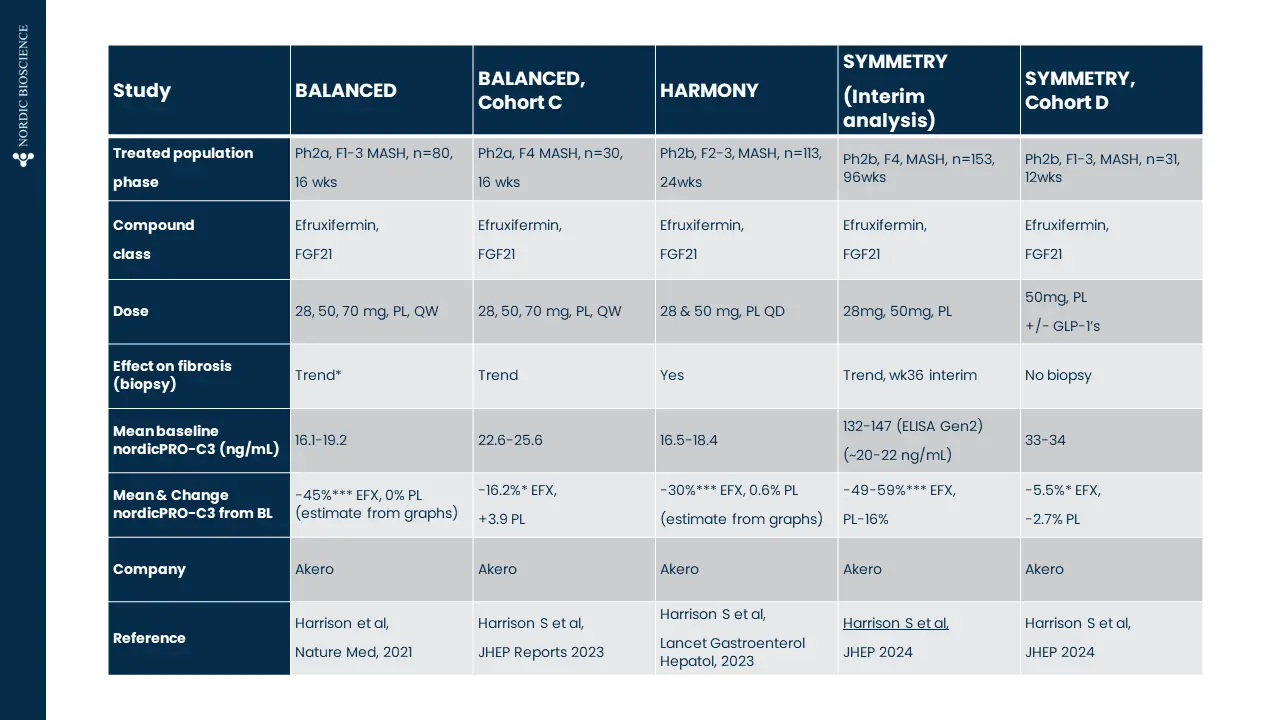
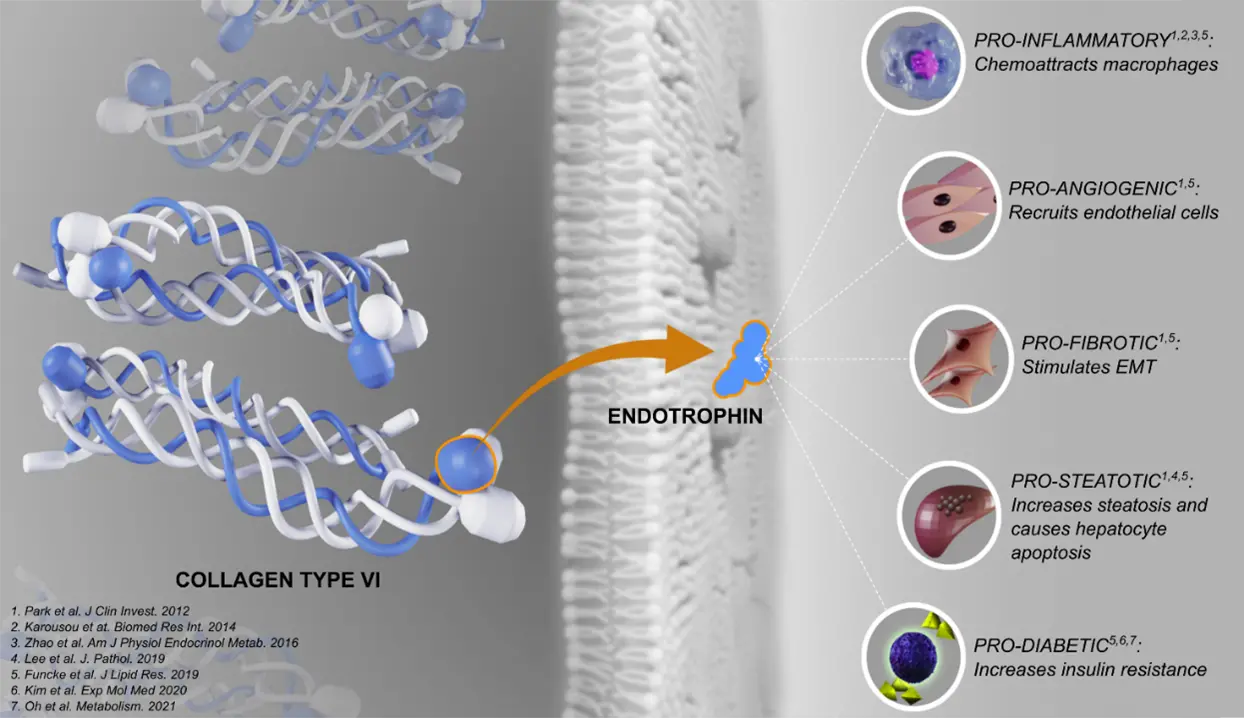
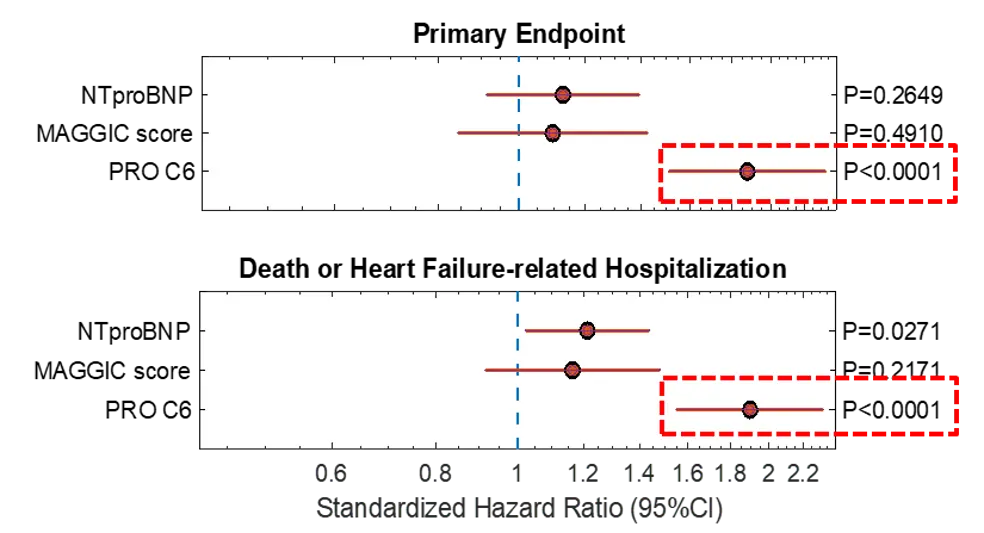
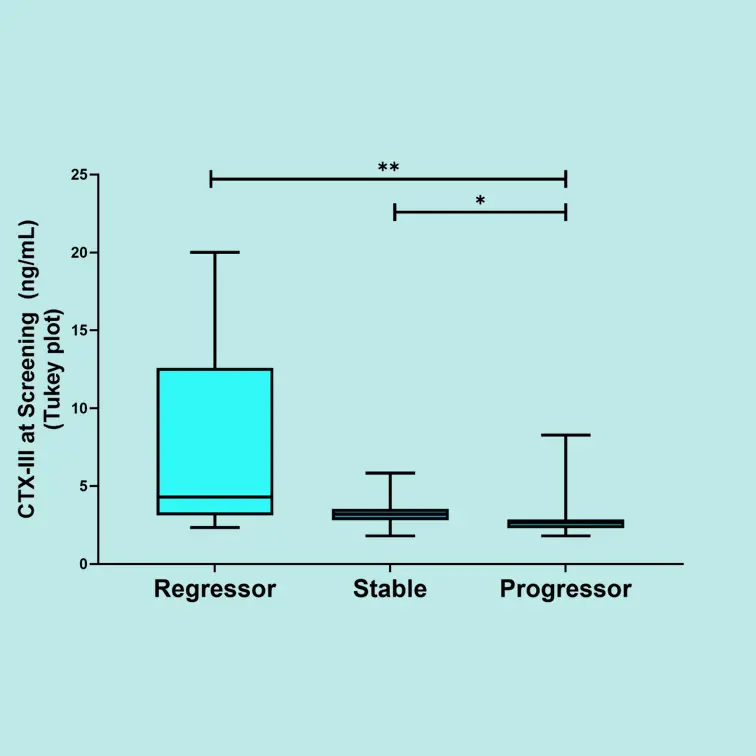
FIB-NIT™ (Fibrosis Non-Invasive Tests) is an innovative panel that utilizes biomarkers of fibrotic processes.
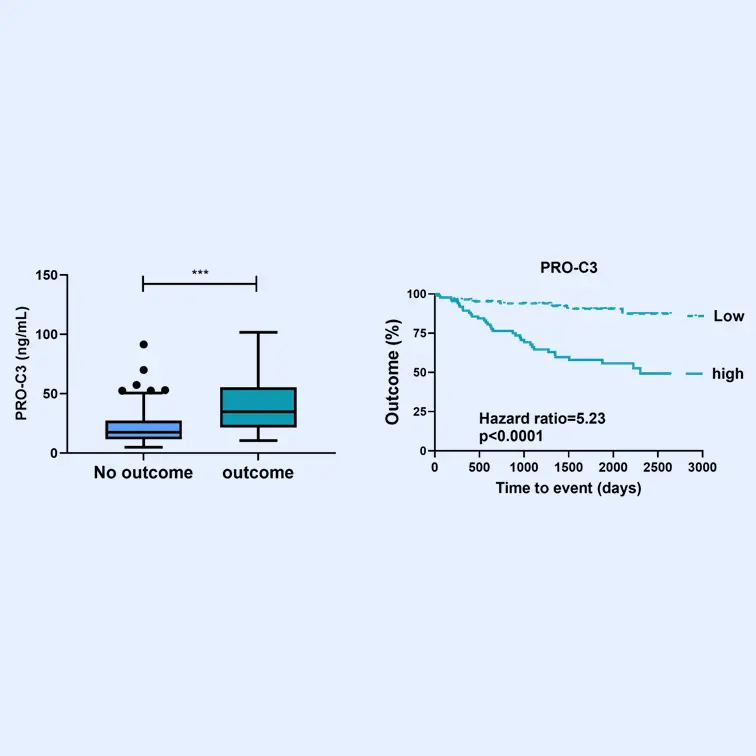
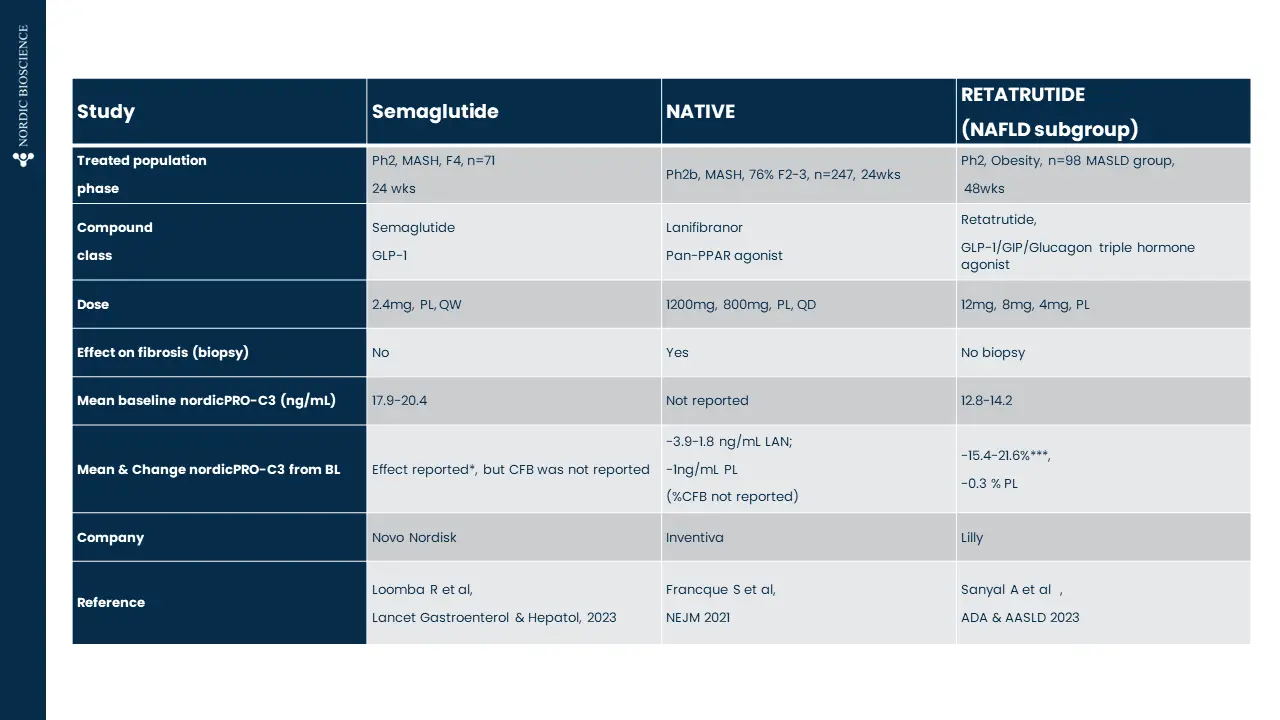
These biomarkers have been proven to associate with superior pharmacodynamic and supportive prognostic performance to investigate dynamics within fibrotic diseases, including understanding drug efficacy in such indications —demonstrated in over 100 publications in relevant and peer-reviewed scientific journals.
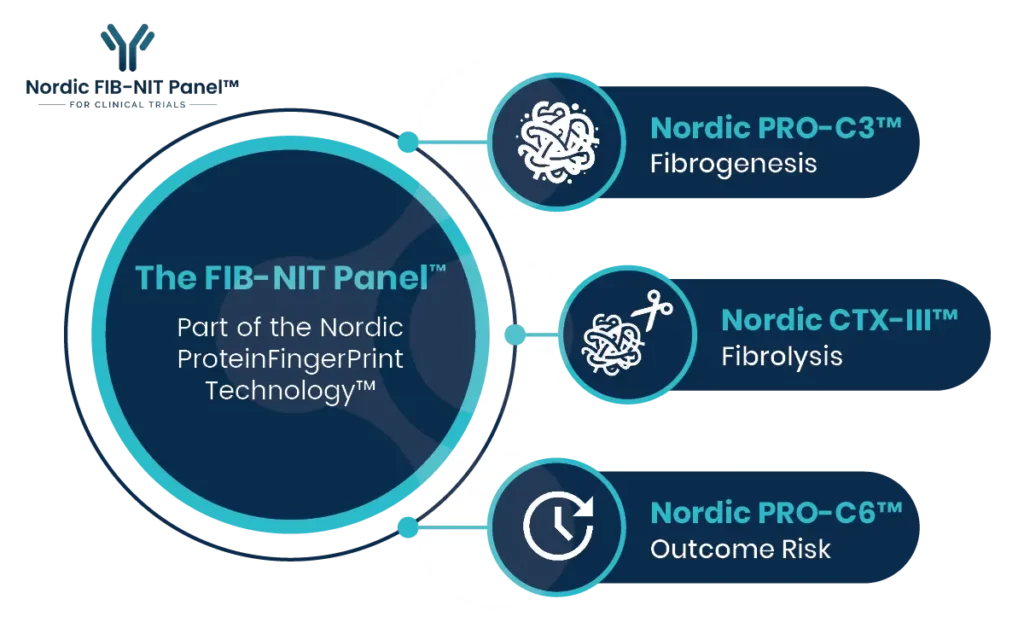
The FIB-NIT™ panel consists of the fibrogenesis nordicPRO-C3™, the fibrolysis nordicCTX-III™, and the outcome risk nordicPRO-C6™ biomarkers.