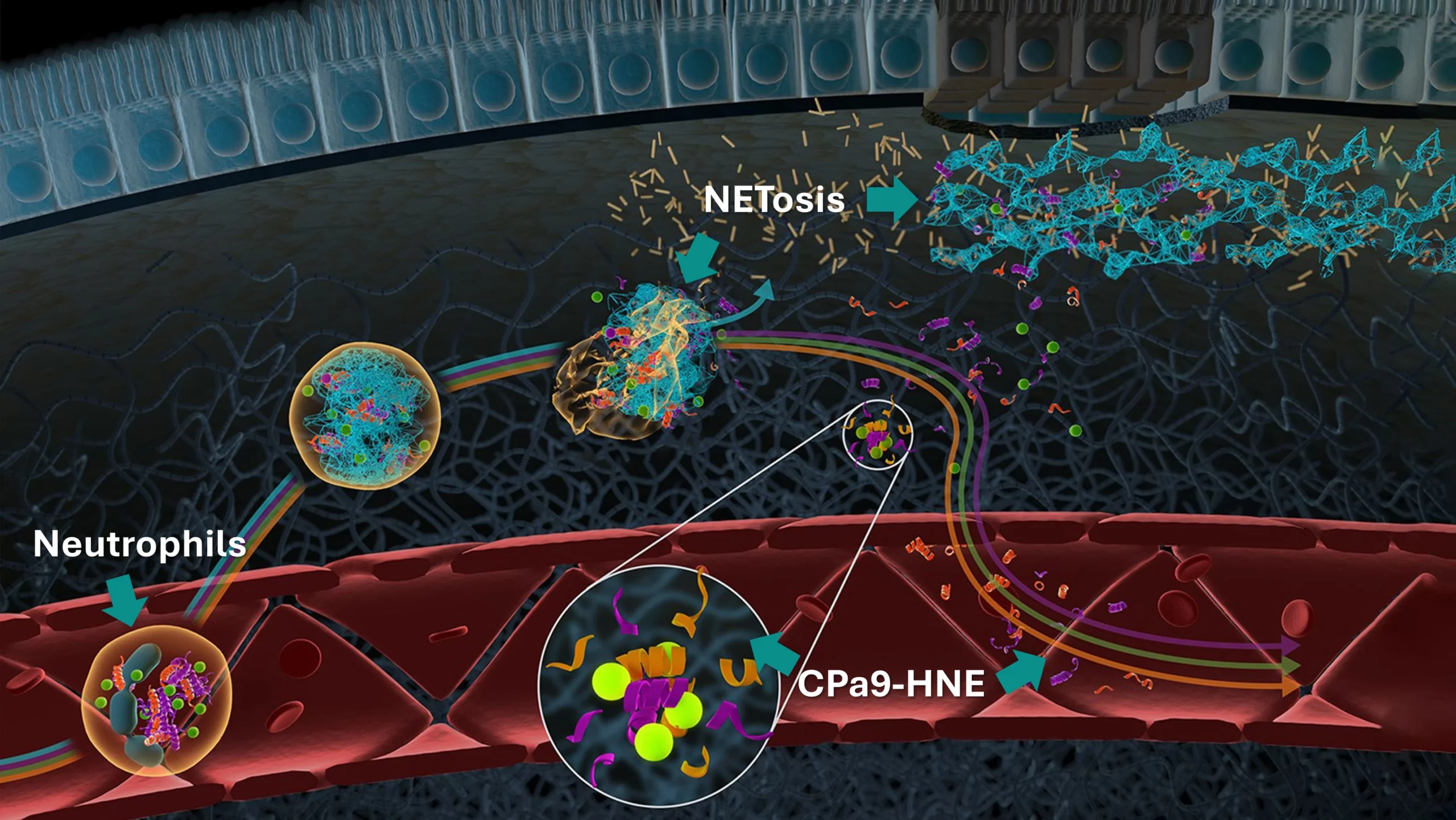
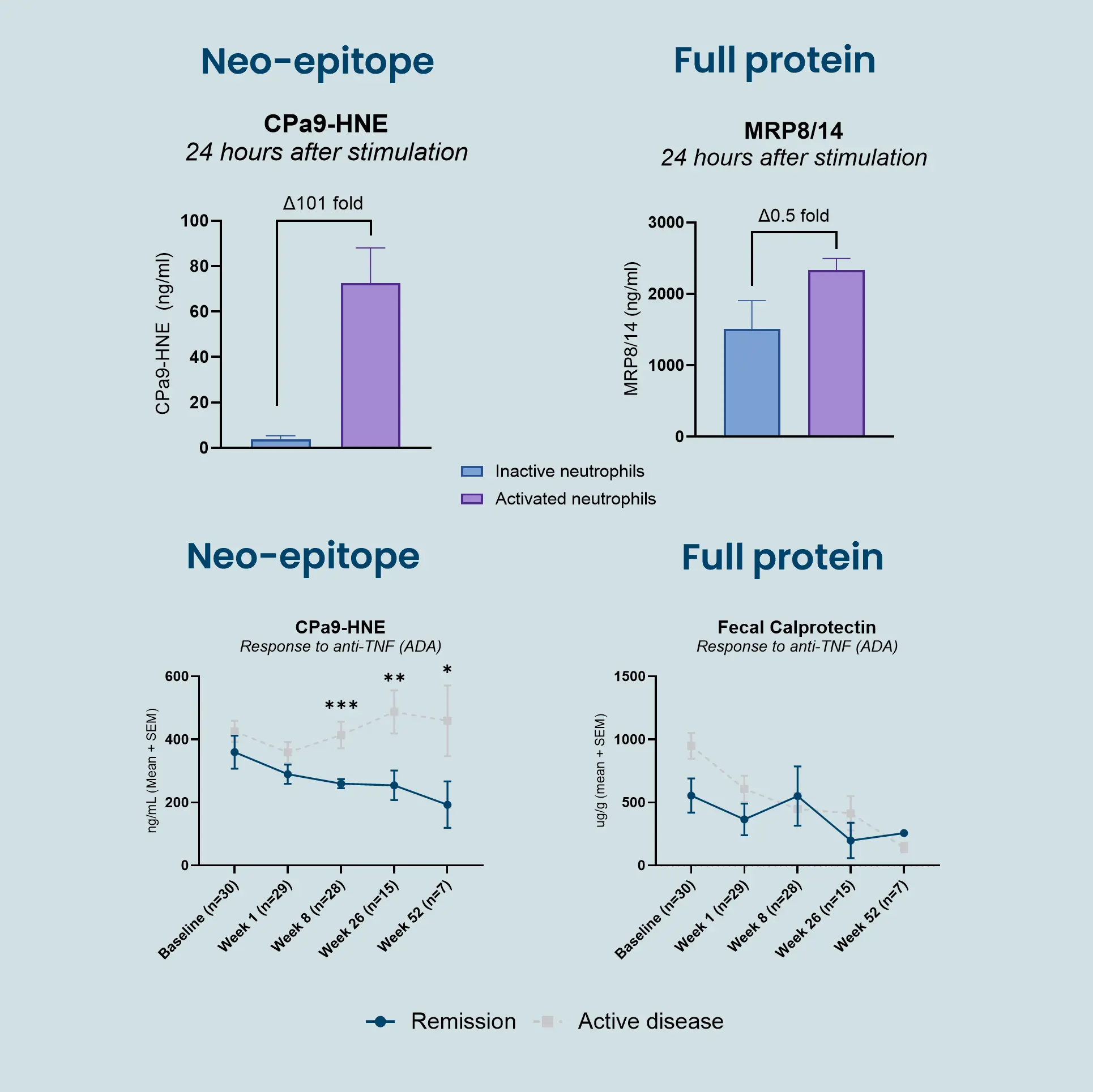
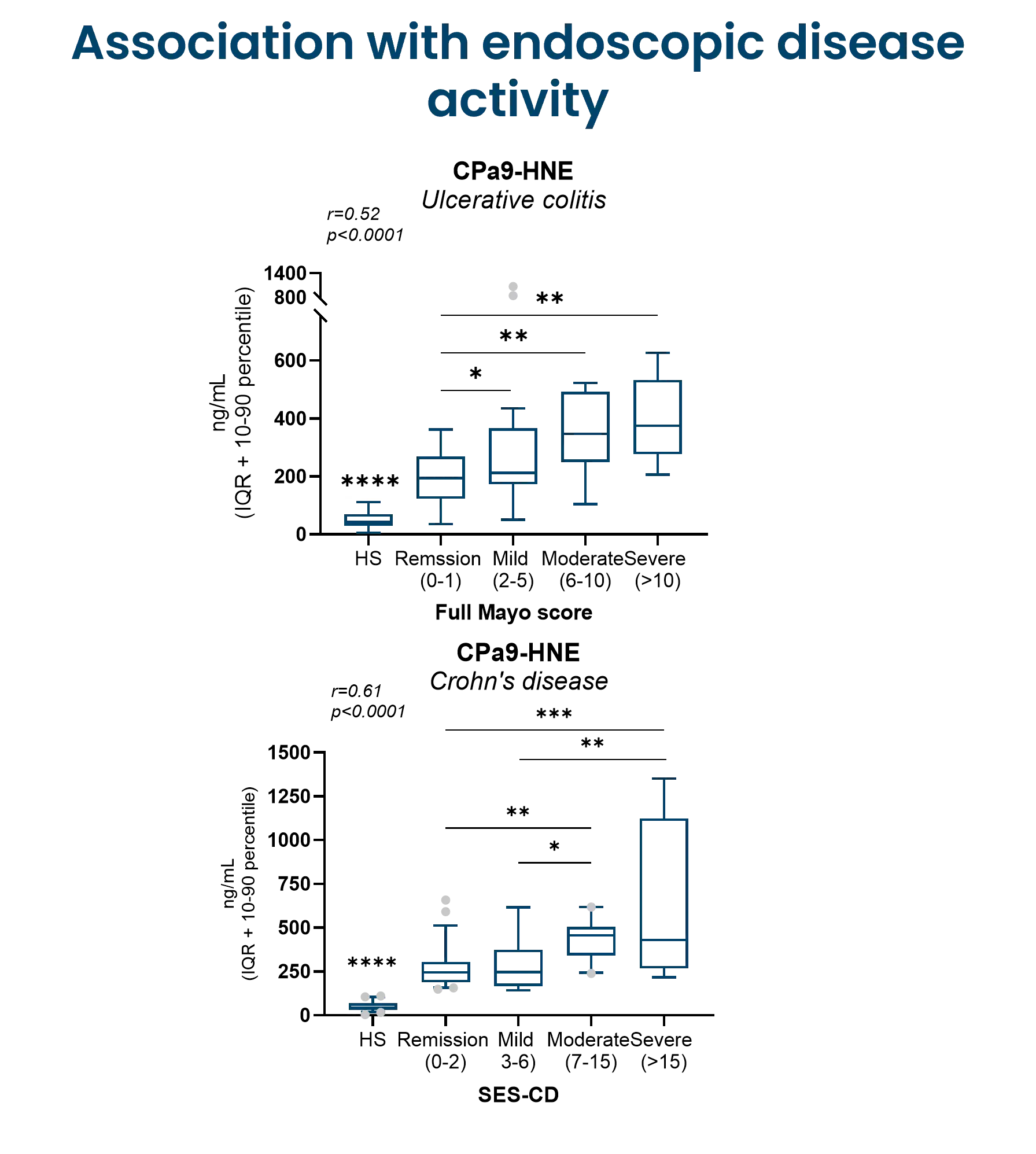
The CPa9-HNE (nordicCPa9-HNE™) biomarker provides a measure of true neutrophil activity by capturing the interplay between two specific key neutrophilic intracellular components; the antimicrobial protein calprotectin and the protease human neutrophil elastase (HNE).
Constant activation and recruitment of neutrophils is a common feature in IBD (Crohn’s disease and ulcerative colitis). Their infiltration into the intestinal tissue has been correlated to disease activity, where they induce mucosal damage through the release of neutrophil extracellular traps (NETosis). Therefore, measuring neutrophil activity using CPa9-HNE provides valuable insight into disease progression and inflammatory burden, potentially guiding the development and evaluation of novel therapeutic interventions, as further encouraged by the Letter of Support from the FDA.