A drug development route for success is key but often challenging and full of uncertainties. Strategies that can increase the likelihood of success are vital. The FDA and EMA recognize that biomarker-based drug development strategies, in combination with translation research, could be the solution. This approach offers a biology-focused pathway that not only reduces time and cost but also enhances the overall likelihood of success.
Translational Models in Pulmonary Fibrosis
Translational biomarkers in pulmonary research
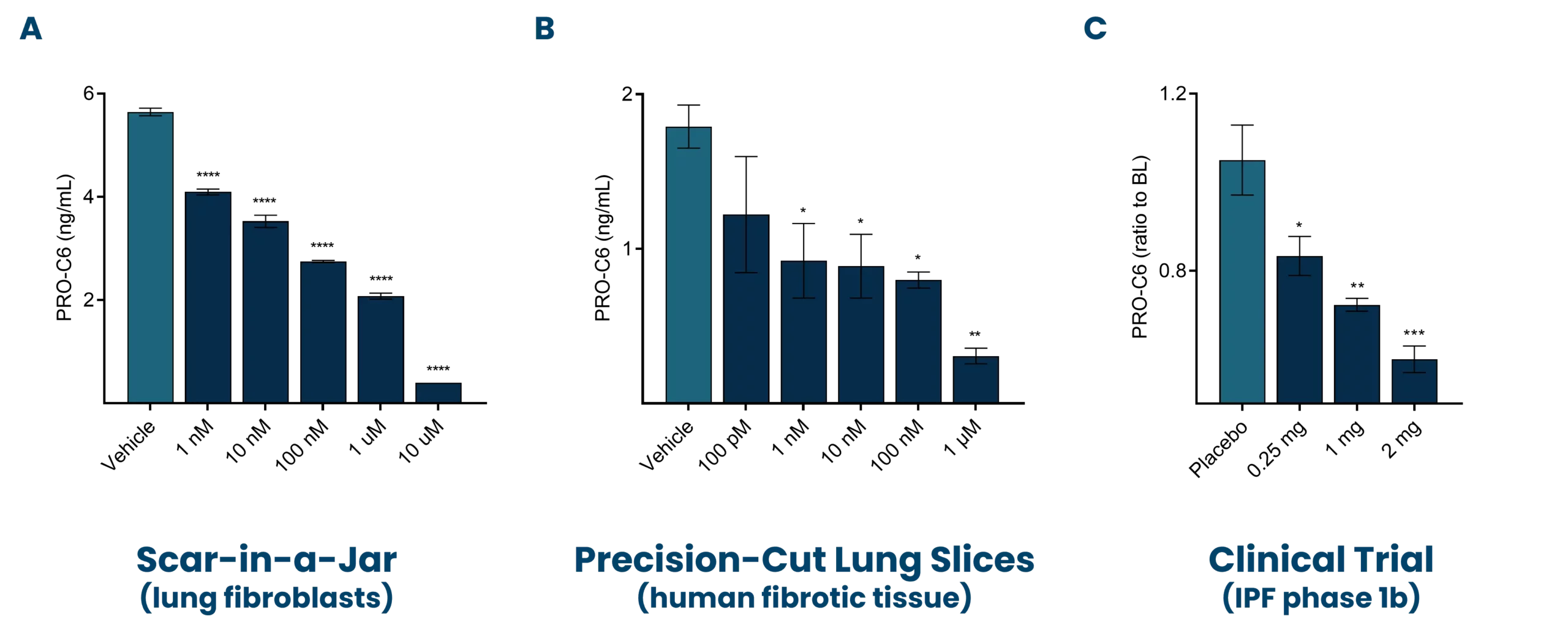
A major cornerstone of ours is to combine translational models with clinically validated biomarkers to enhance drug development. For this purpose, we have assessed the ability of our biomarkers in bridging the gap between pulmonary fibrosis patients and various model systems. Our investigations have revealed that ProteinFingerPrint™ biomarkers, such as nordicPRO-C6™ (PRO-C6, also known as Endotrophin), show dose-dependent modulation in response to antifibrotic treatment. We assessed both in the blood of idiopathic pulmonary fibrosis (IPF) patients and in the supernatant of pulmonary fibrosis models (Figure 1) [7,8,9].
In vitro—Scar-in-a-Jar (SiaJ)
The prolonged Scar-in-a-Jar is an innovative model that utilizes macromolecular crowding to enhance the formation, maturation, and deposition of the extracellular matrix in vitro. The extracellular matrix is essential for providing structural support to cells and regulating tissue repair and regeneration by serving as a reservoir for growth factors and cytokines.
Understanding the dynamics of the extracellular matrix is crucial for developing therapeutic strategies for fibrosis. By employing macromolecular crowding, the model gains a three-dimensional like structure and increased complexity, making it a suitable translational model. This modulation demonstrates the model’s effectiveness for screening antifibrotic effects directly on hepatic stellate cells.
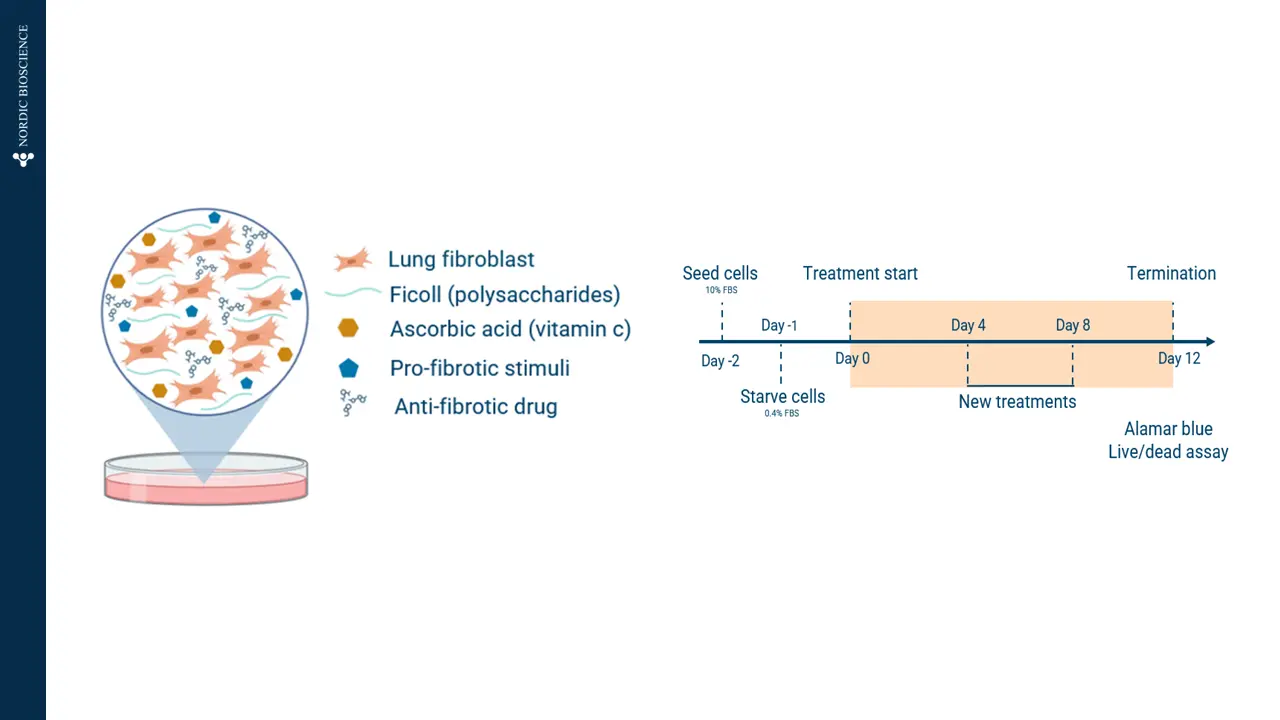 Figure 2. Prolonged Scar-in-a-Jar model in vitro.
Figure 2. Prolonged Scar-in-a-Jar model in vitro.
The figures show how PRO-C3 (nordicPRO-C3™) (formation of type III collagen; PRO-C3) and PRO-C6 (nordicPRO-C6™) (formation of type VI collagen; PRO-C6) increase when stimulating lung fibroblasts with a fibrotic cocktail.
In addition, PRO-C3 (nordicPRO-C3™) and PRO-C6 (nordicPRO-C6™) levels are affected and can be decreased when stimulating the fibroblasts and treating them with Omipalisib.
This modulation shows that the model is applicable for screening of antifibrotic effects directly on the fibroblasts.
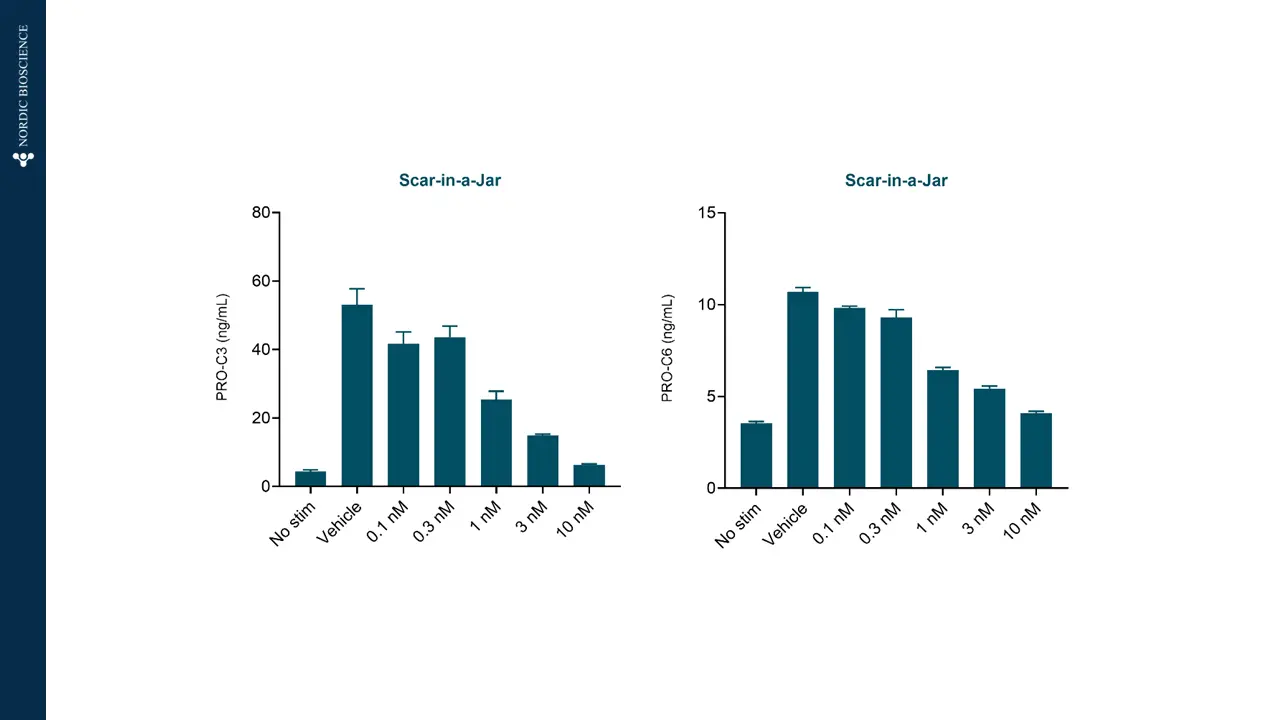 Figure 3. Scar-in-a-Jar can screen antifibrotic effects.
Figure 3. Scar-in-a-Jar can screen antifibrotic effects.
In vivo—bleomycin model
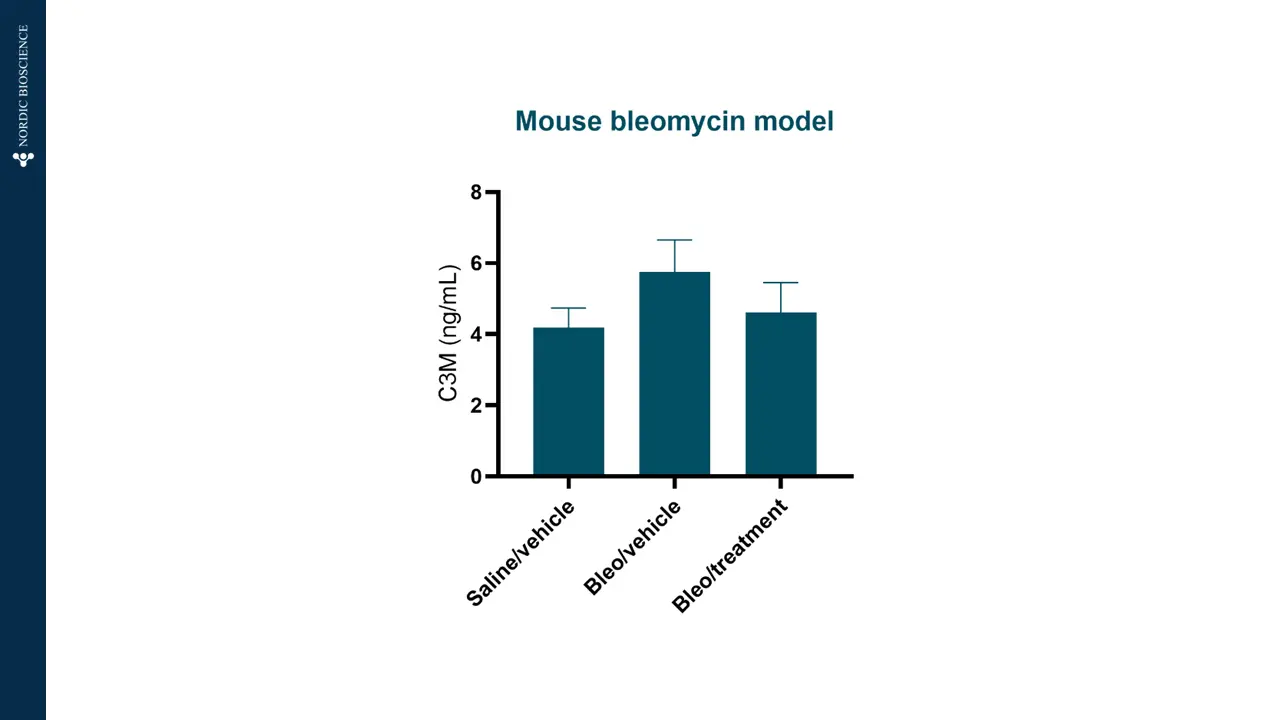
The bleomycin model has become an important translational model for pulmonary fibrosis. The model allows us to study pulmonary fibrosis in vivo. To assess efficacy, Nordic ProteinFingerPrint™ biomarkers are measured in the blood of patients.
The graph shows how C3M (type III collagen degraded by MMP) is increased in bleomycin-treated mice compared to saline, meaning that fibrotic processes are present in the model.
Also, C3M levels are lowered to the same level as saline when adding an antifibrotic treatment to the bleomycin-treated mice. The treatment effectively reduced collagen degradation and fibrosis, restoring the tissue environment.
Get in touch
Nordic Bioscience’s assays and services are research use only products and services and do not qualify for medical or diagnostic purposes.