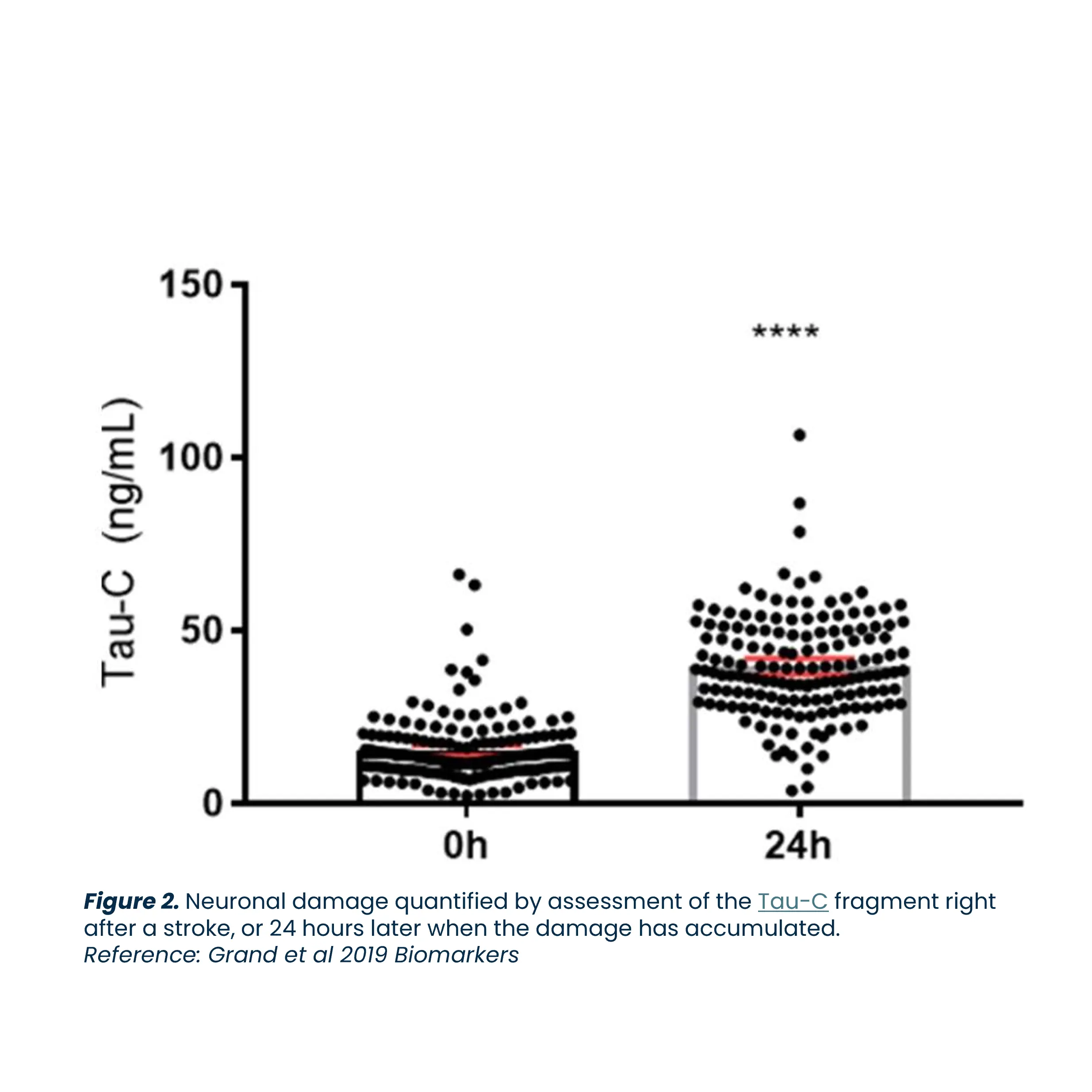
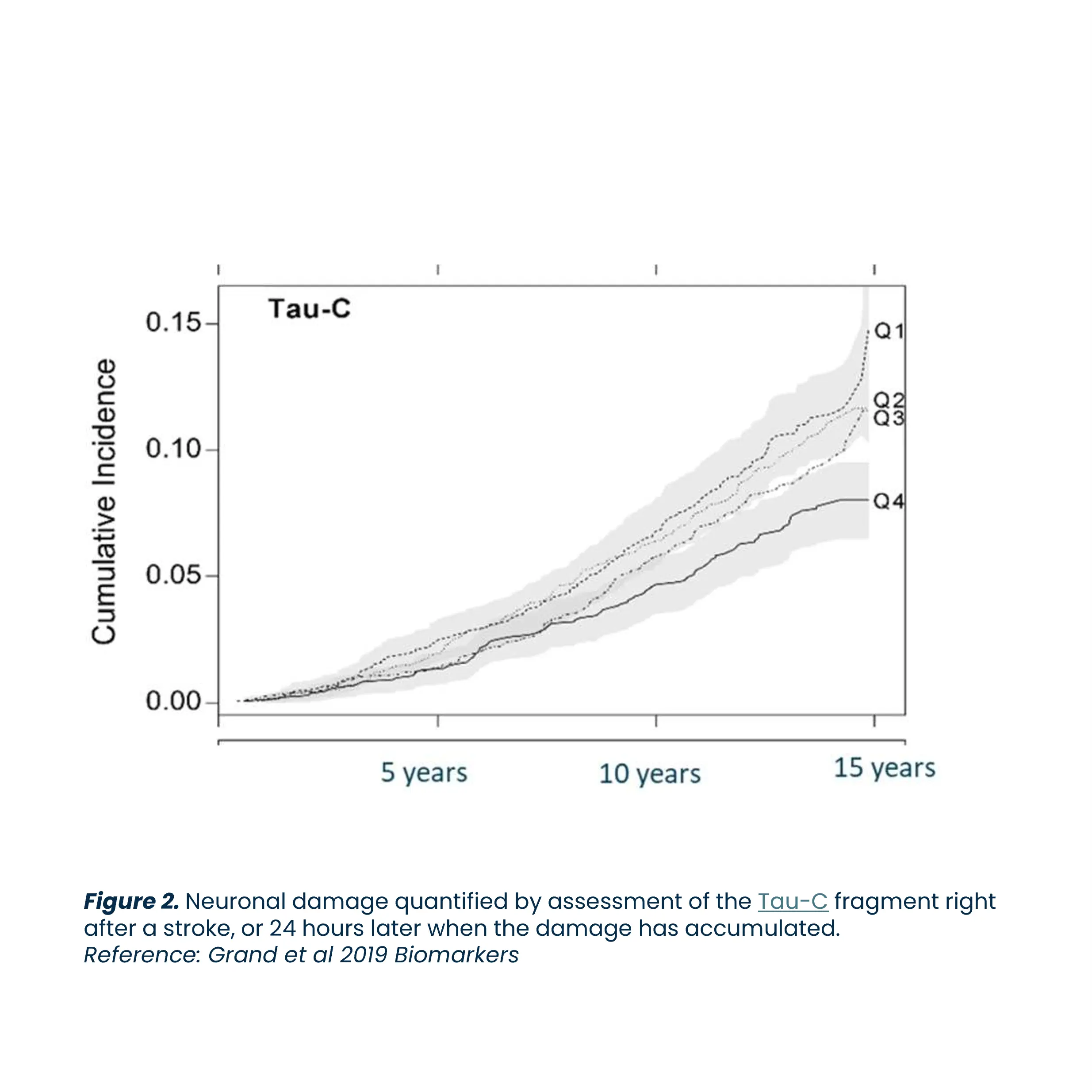
Alzheimer’s disease is a neurodegenerative disorder that leads to progressive cognitive decline and is the most common cause of dementia. The disease involves the loss of neurons in the brain, which impairs memory, thinking, and behavior. Alzheimer’s disease is a major global health issue, affecting approximately 40-50 million people worldwide, and the number of individuals with the disease is expected to reach 100 million by 2050.
Biomarkers are key to advancing Alzheimer’s research. They enhance diagnosis, predict prognosis, evaluate treatment responses, and identify potential therapeutic targets, driving the development of effective treatments.