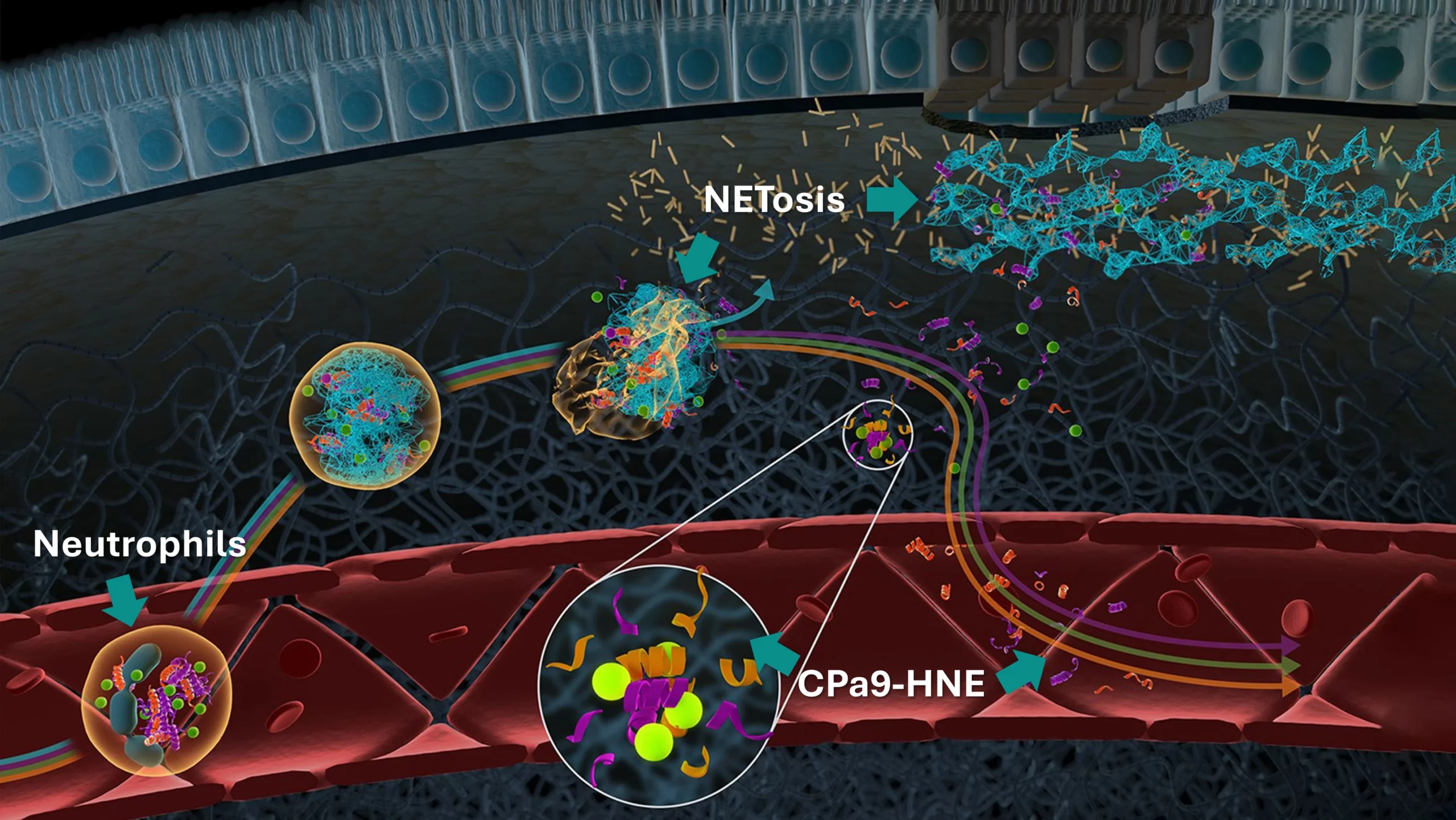
Inflammatory bowel disease (IBD) is a chronic, relapsing, and remitting inflammatory disorder encompassing two main clinical entities: ulcerative colitis (UC) and Crohn‘s disease (CD). The etiology of IBD involves a complex interplay between genetic susceptibility, environmental factors, and excessive immune response to the intestinal microbiota—mediated by immune cells such as neutrophils, macrophages and T-cells.
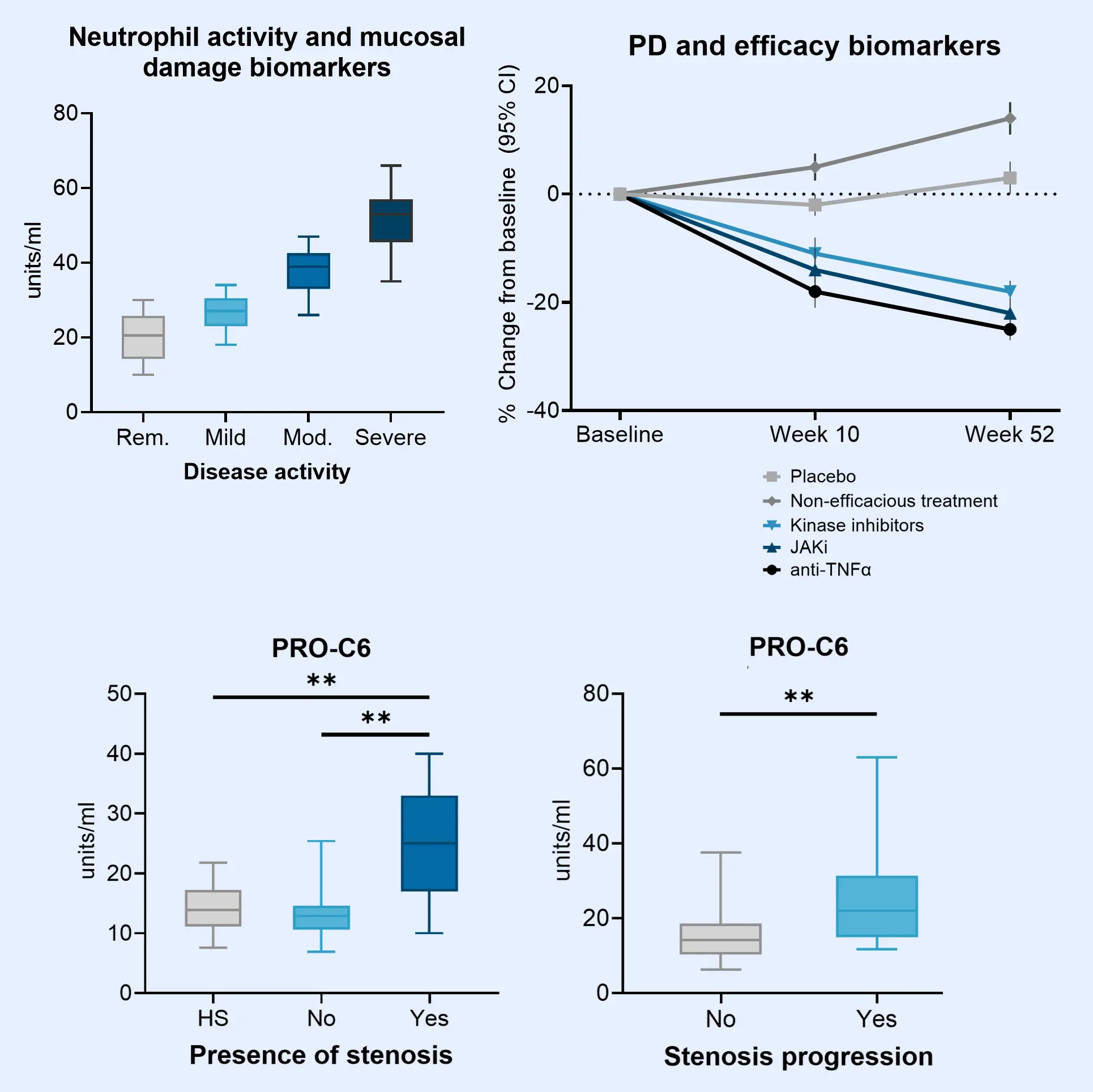
A hallmark of IBD is dysregulated proteolytic activity that throws off the balance of extracellular matrix (ECM) remodeling, particularly collagen turnover within the mucosa and submucosa. This disruption in ECM remodeling contributes to key pathological features, including epithelial barrier dysfunction, chronic inflammation, and fibrosis.
Generally, disrupted organ function in IBD is quantified through low patient compliance fecal calprotectin measurements. However, because of increased collagen turnover, ECM-based biomarkers allow researchers to measure calprotectin, inflammation, epithelial damage and fibrosis directly in the blood, improving patient compliance and data availability.