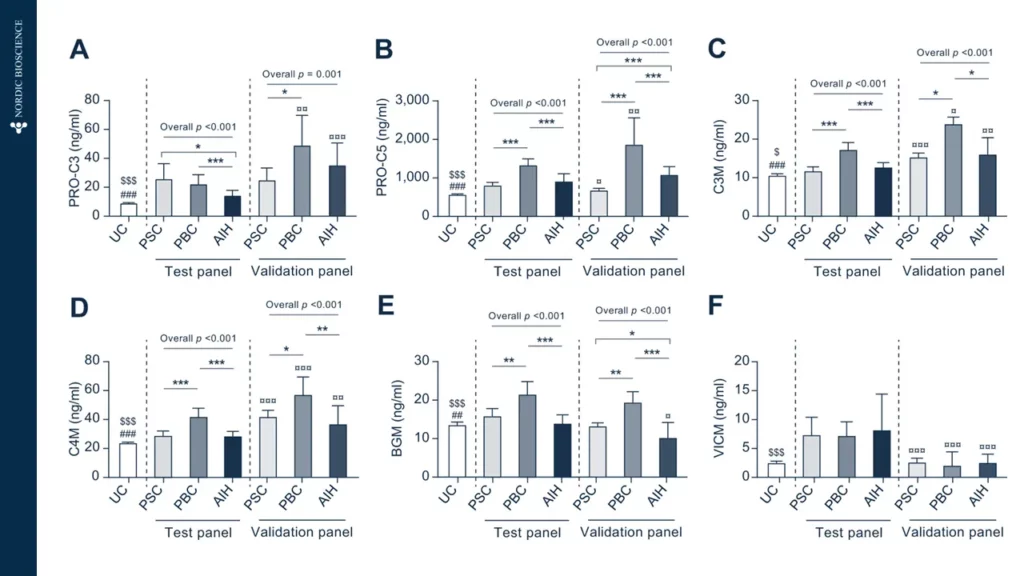
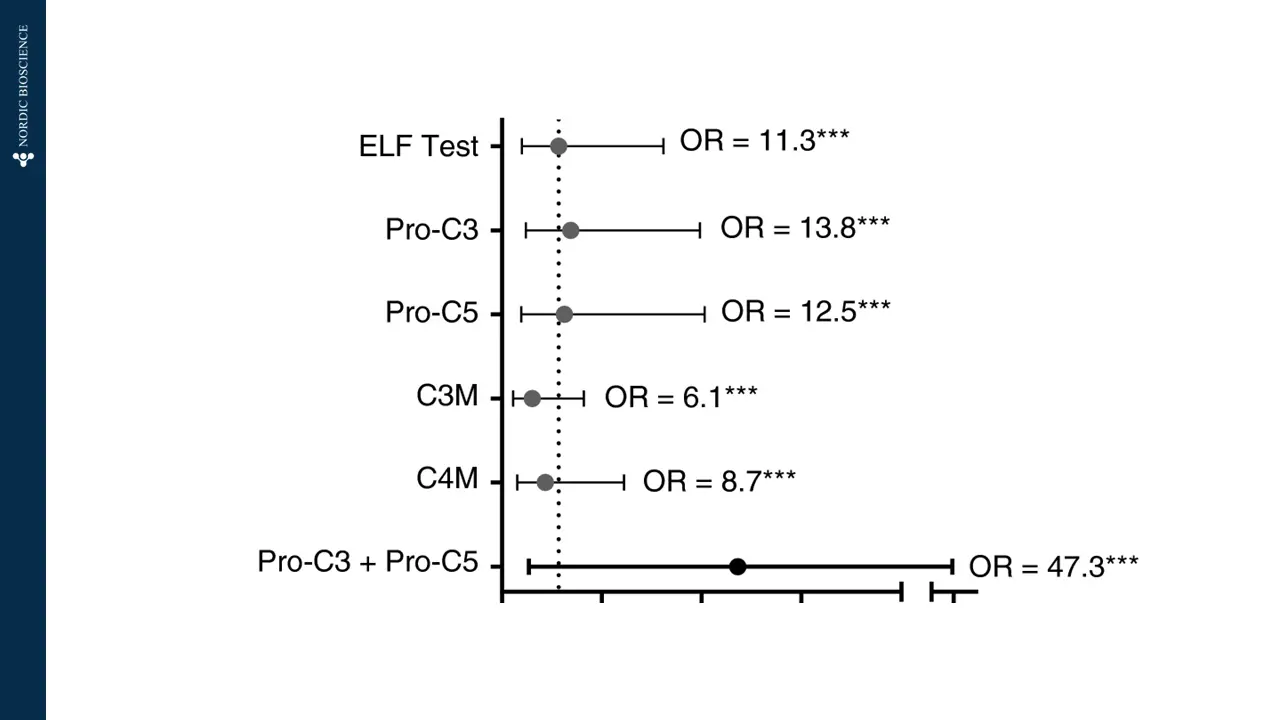
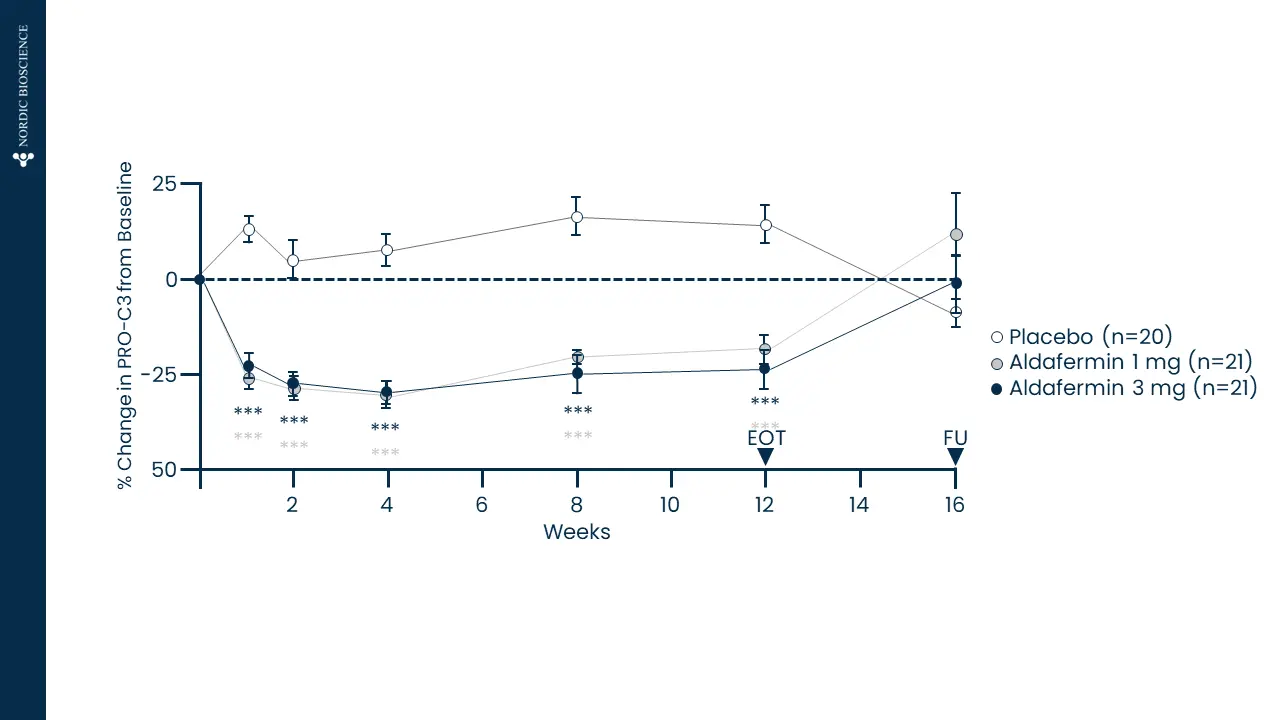
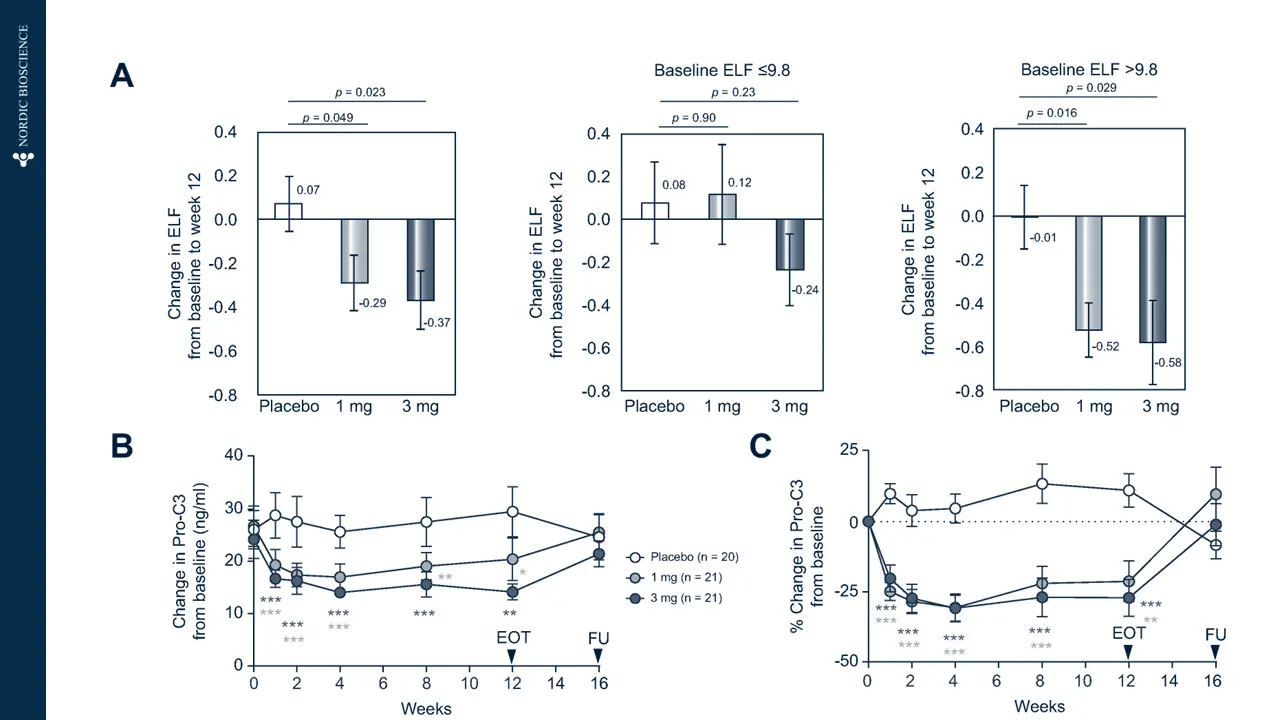
Immune-mediated liver diseases necessitate new biomarkers that can accurately assess the risk of disease progression and the likelihood of response to treatment. Fibrosis is a key histologic feature of immune-mediated liver diseases, such as autoimmune hepatitis (AIH), primary biliary cholangitis (PBC) and primary sclerosing cholangitis (PSC).
Did you know?
Non-invasive biomarkers have the potential to revolutionize the screening and treatment of immune-mediated liver diseases by providing a novel approach to determining and monitoring the severity of liver fibrosis and the progression of fibrotic processes.
Therefore, the development of such biomarkers has a large potential to improve the current methods of screening and the treatment of immune-mediated liver diseases.