The Enhanced Liver Fibrosis (ELF™) test is a noninvasive blood (serum) lab test, which combines three direct ECM biomarkers: hyaluronic acid (HA), procollagen III aminoterminal peptide (PIIINP), and tissue inhibitor of matrix metalloproteinase 1 (TIMP-1), into a validated composite score. The ELF™ score helps clinicians and researchers assess fibrosis severity and risk in metabolic and chronic liver diseases, including MASLD/NAFLD.
The ELF™ Score
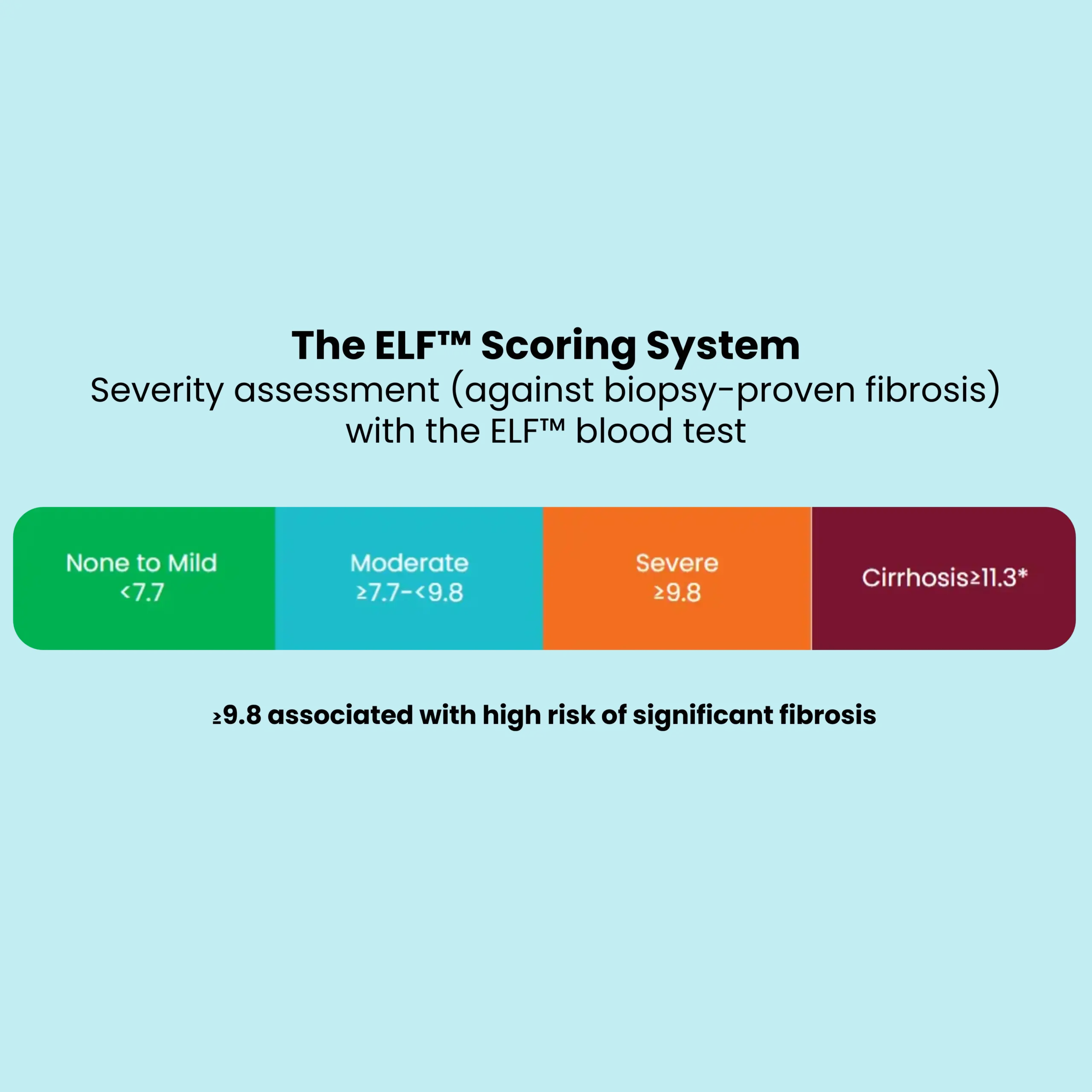
The ELF™ score has been well-validated against biopsy-proven fibrosis across a range of chronic liver diseases (CLD) in both adult and pediatric populations. The three direct markers of the ELF test provide complementary information, and the combined score outperforms both the individual markers and simple scores such as APRI or FIB-4.
The three analytes of the ELF™ are measured automatically, and the software calculates and reports a unitless numeric score. Increased ELF scores are linked to both biopsy-proven fibrosis and prognosis for clinically significant outcomes (Figure 1).