1. Blair, J. P. M., Bager, C., Platt, A., Karsdal, M., & Bay-Jensen, A.-C. (2019). Identification of pathological RA endotypes using blood-based biomarkers reflecting tissue metabolism. A retrospective and explorative analysis of two phase III RA studies. PloS One, 14(7), e0219980. https://doi.org/10.1371/journal.pone.0219980
2. Hannani, M. T., Thudium, C. S., Karsdal, M. A., Ladel, C., Mobasheri, A., Uebelhoer, M., Larkin, J., Bacardit, J., Struglics, A., & Bay-Jensen, A.-C. (2024). From biochemical markers to molecular endotypes of osteoarthritis: a review on validated biomarkers. Expert Review of Molecular Diagnostics, 24(1–2), 23–38. https://doi.org/10.1080/14737159.2024.2315282
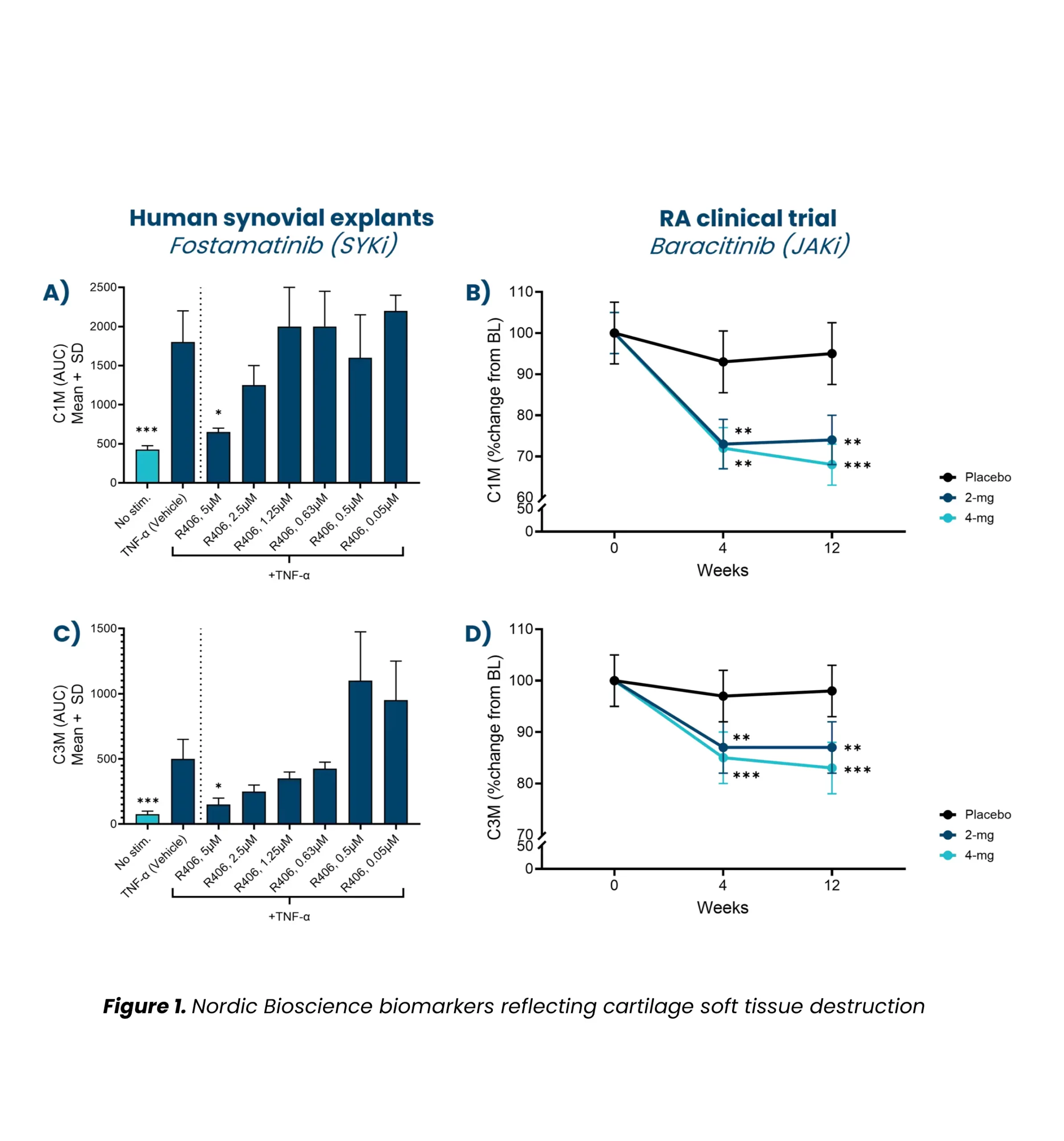
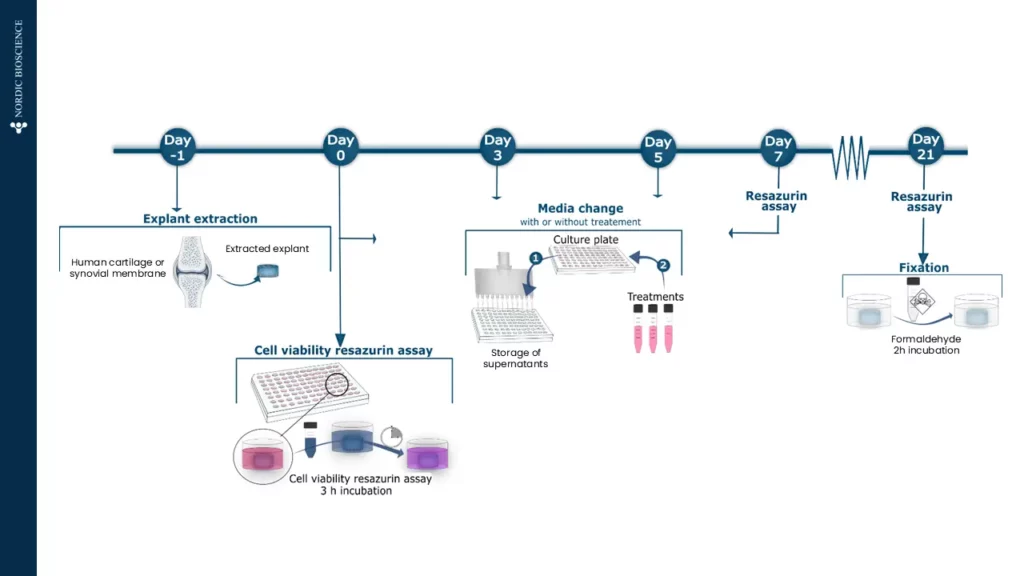
3. Kjelgaard-Petersen, C. F., Platt, A., Braddock, M., Jenkins, M. A., Musa, K., Graham, E., Gantzel, T., Slynn, G., Weinblatt, M. E., Karsdal, M. A., Thudium, C. S., & Bay-Jensen, A.-C. (2018). Translational Biomarkers and Ex Vivo Models of Joint Tissues as a Tool for Drug Development in Rheumatoid Arthritis. Arthritis & Rheumatology (Hoboken, N.J.), 70(9), 1419–1428. https://doi.org/10.1002/art.40527
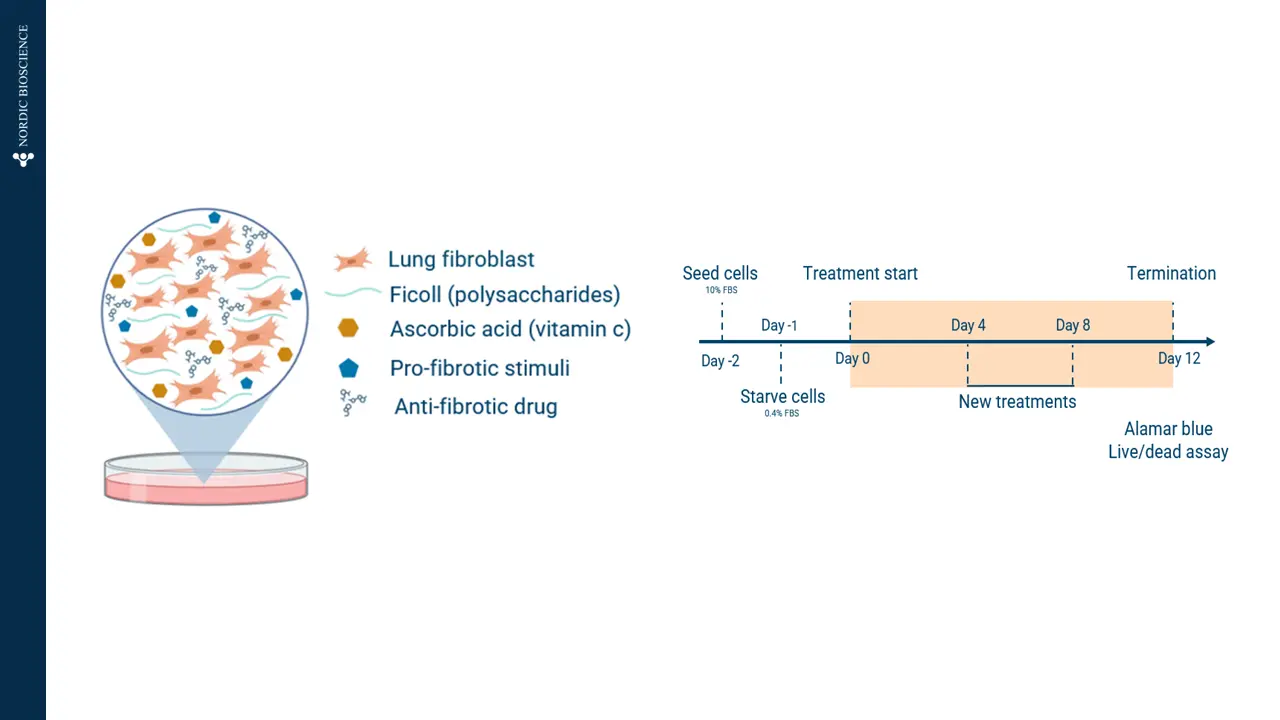
4. Madsen, S. F., Madsen, S. S., Madrid, A. S., Andersen, M. R., Bay-Jensen, A.-C., & Thudium, C. S. (2024). Fibrotic remodeling in joint diseases: induction and inhibition of fibrosis in fibroblast-like synoviocytes. Translational Medicine Communications, 9(1), 18. https://doi.org/10.1186/s41231-024-00180-0
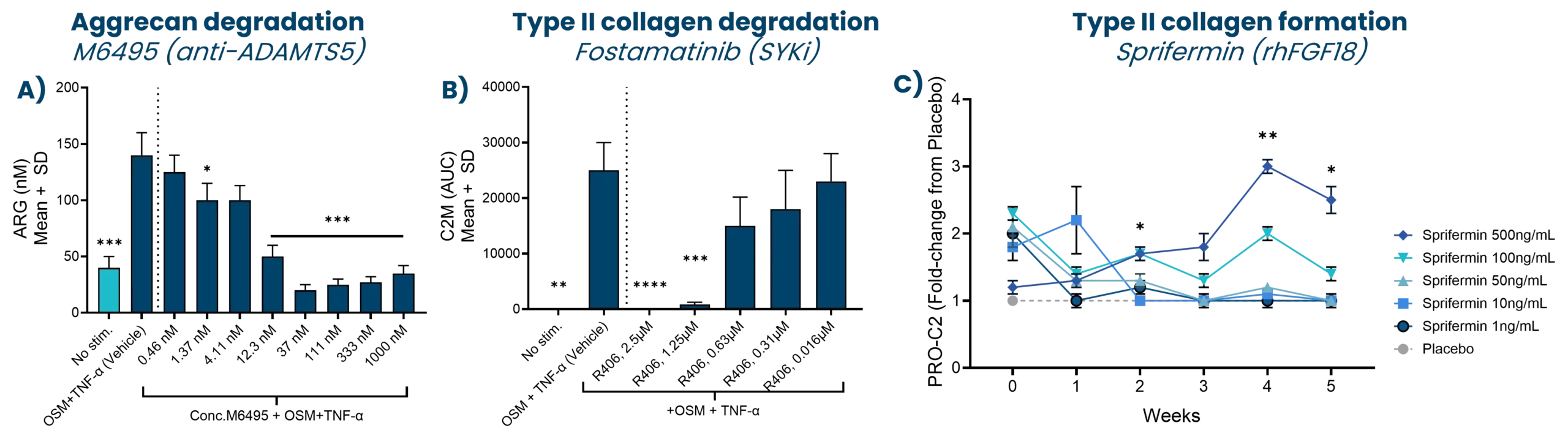
5. Reker, D., Siebuhr, A. S., Thudium, C. S., Gantzel, T., Ladel, C., Michaelis, M., Aspberg, A., Berchtold, M. W., Karsdal, M. A., Gigout, A., & Bay-Jensen, A. C. (2020). Sprifermin (rhFGF18) versus vehicle induces a biphasic process of extracellular matrix remodeling in human knee OA articular cartilage ex vivo. Scientific Reports, 10(1), 6011. https://doi.org/10.1038/s41598-020-63216-z
6. Siebuhr, A. S., Wang, J., Karsdal, M., Bay-Jensen, A.-C., Y, J., & Q, Z. (2012). Matrix Metalloproteinase-dependent turnover of cartilage, synovial membrane, and connective tissue is elevated in rats with collagen induced arthritis. Journal of Translational Medicine, 10(1), 195. https://doi.org/10.1186/1479-5876-10-195
7. Siebuhr, A. S., Werkmann, D., Bay-Jensen, A.-C., Thudium, C. S., Karsdal, M. A., Serruys, B., Ladel, C., Michaelis, M., & Lindemann, S. (2020). The Anti-ADAMTS-5 Nanobody® M6495 Protects Cartilage Degradation Ex Vivo. International Journal of Molecular Sciences, 21(17). https://doi.org/10.3390/ijms21175992
8. Thudium, C. S., Bay-Jensen, A. C., Cahya, S., Dow, E. R., Karsdal, M. A., Koch, A. E., Zhang, W., & Benschop, R. J. (2020). The Janus kinase 1/2 inhibitor baricitinib reduces biomarkers of joint destruction in moderate to severe rheumatoid arthritis. Arthritis Research & Therapy, 22(1), 235. https://doi.org/10.1186/s13075-020-02340-7