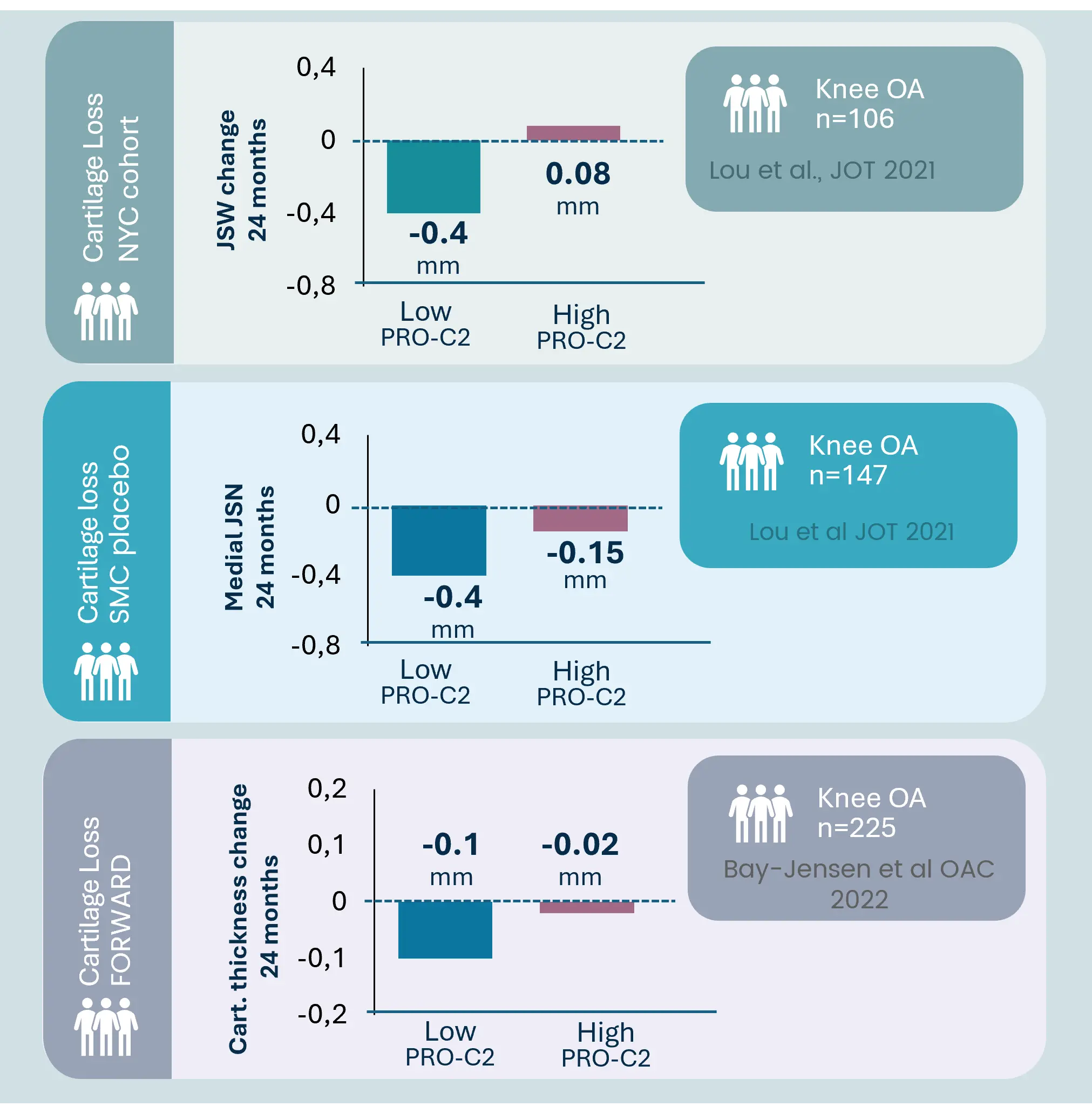
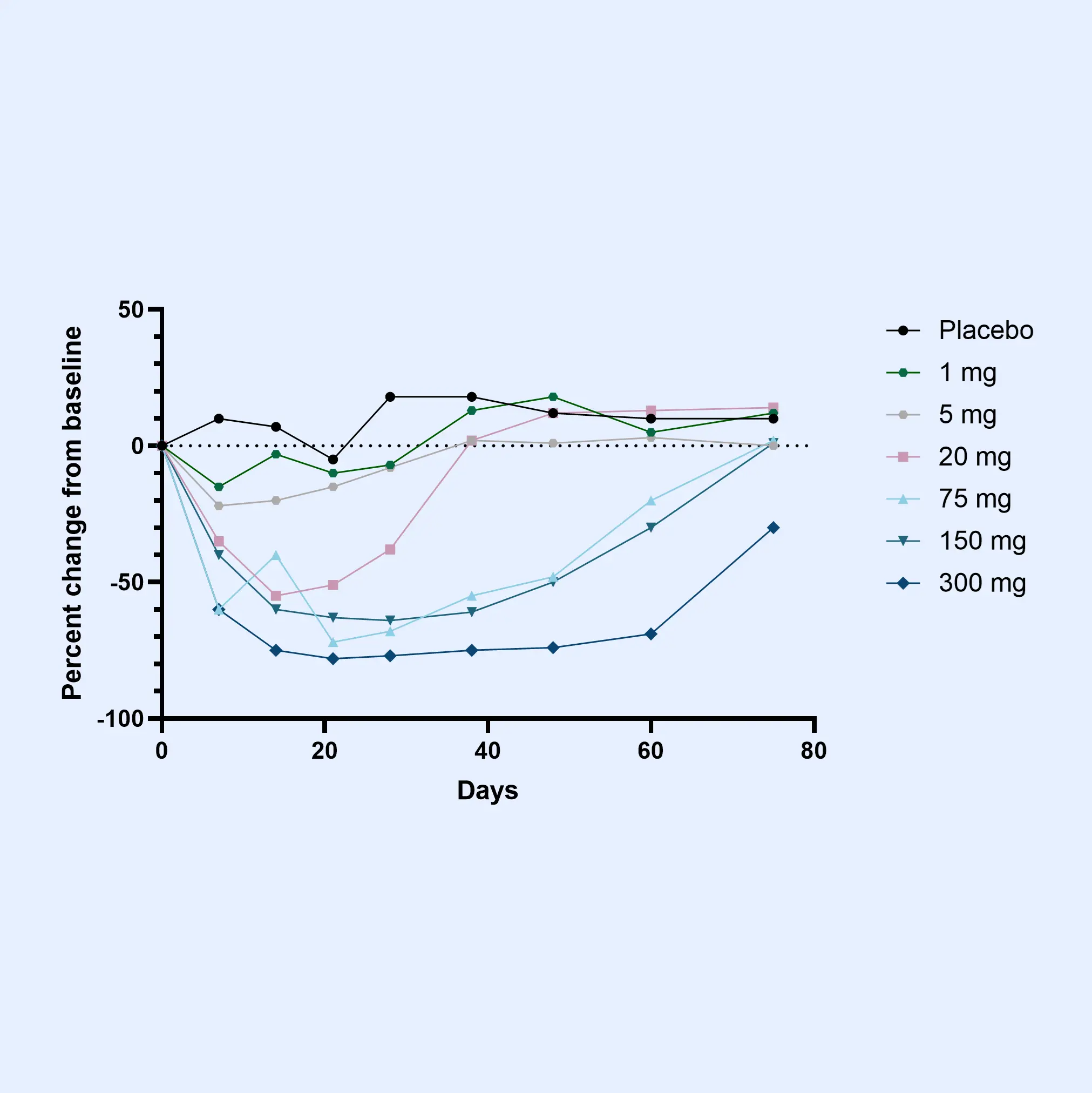
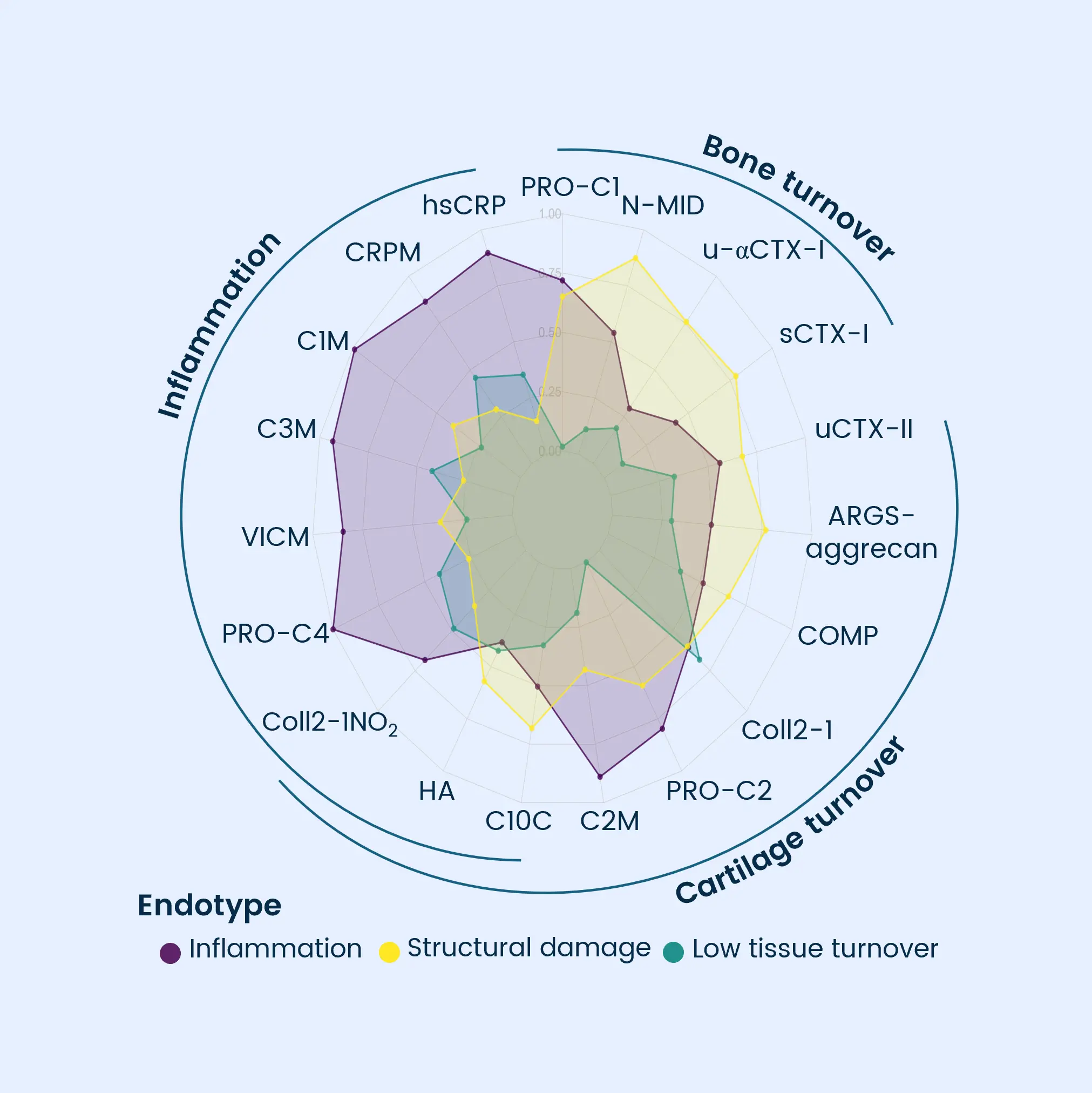
Osteoarthritis is characterized by progressive joint degradation and abnormal remodeling of cartilage and bone tissue. The Nordic ProteinFingerPrint Technology™ provides extracellular matrix (ECM) biomarkers specifically designed to quantify the synthesis and degradation of critical joint components such as collagen and proteoglycans in osteoarthritic joints.
These biomarkers facilitate early patient stratification, enabling the identification and selection of individuals likely to experience rapid disease progression for inclusion in clinical trials. The primary endpoints in osteoarthritis clinical trials often involve demonstrating structural changes and slowing the deterioration of joint function and cartilage integrity as measured by imaging methods or patient-reported outcomes.