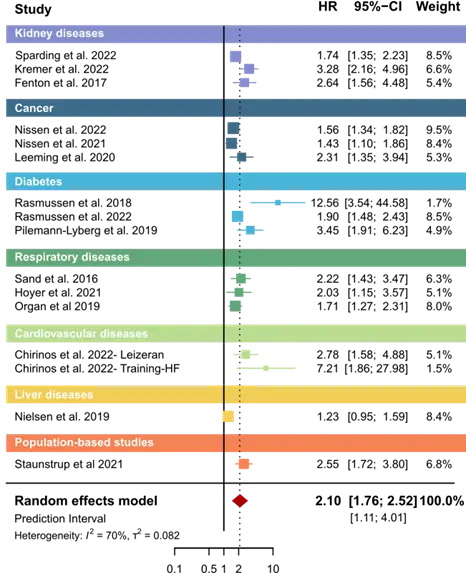
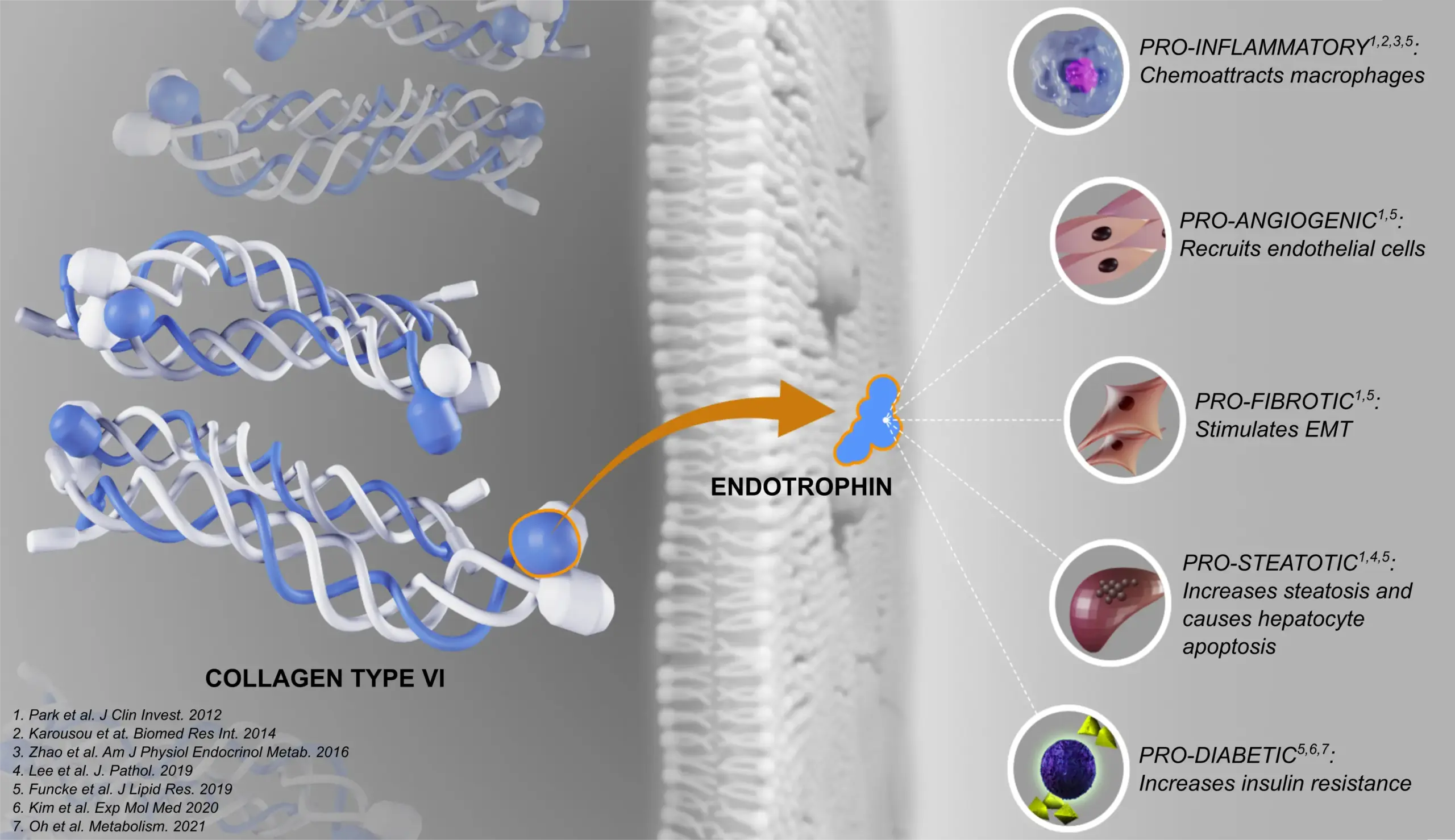
At Nordic Bioscience, our approach to measurement is rooted in decades of innovation and a deep understanding of fibrosis. As the originators of PRO‑C6, we perfected the biomarker over years of clinical trials, built on more than 30 years of expertise in extracellular matrix biomarkers.
Today, PRO-C6 is a well-known name for providing data that is both precise and actionable. PRO-C6 is available in three versions, each with distinct applications:
- NordicPRO-C6™ – Our standard research use only (RUO) assay, measured in our CAP/CLIA-certified lab as an exploratory endpoint or pharmacodynamic biomarker in phase I through phase IV clinical trials. It is available as part of our biomarker testing services and not for diagnostic use.
- NordicPRO-C6™ (manual ELISA kit for human use) – New! The kit can now be directly ordered from us. The kit is FOR RESEARCH USE ONLY (RUO) and can only be used in NON-CLINICAL settings. It can not be used IN DIAGNOSTIC PROCEDURES. To learn more or order, click here.
- NordicrPRO-C6™ (manual ELISA kit for use in rat and mouse) – New! The kit can now be directly ordered from us. The kit is FOR RESEARCH USE ONLY (RUO) and can only be used in NON-CLINICAL settings. It can not be used IN DIAGNOSTIC PROCEDURES. To learn more or order, click here.