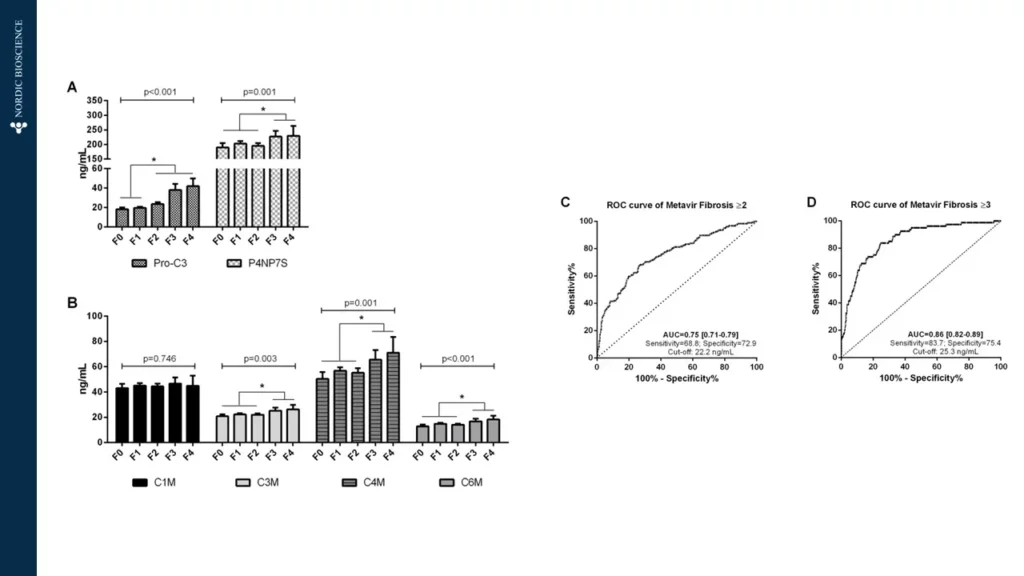
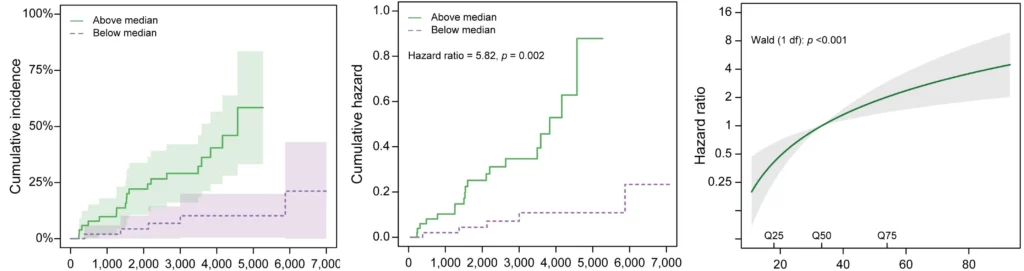
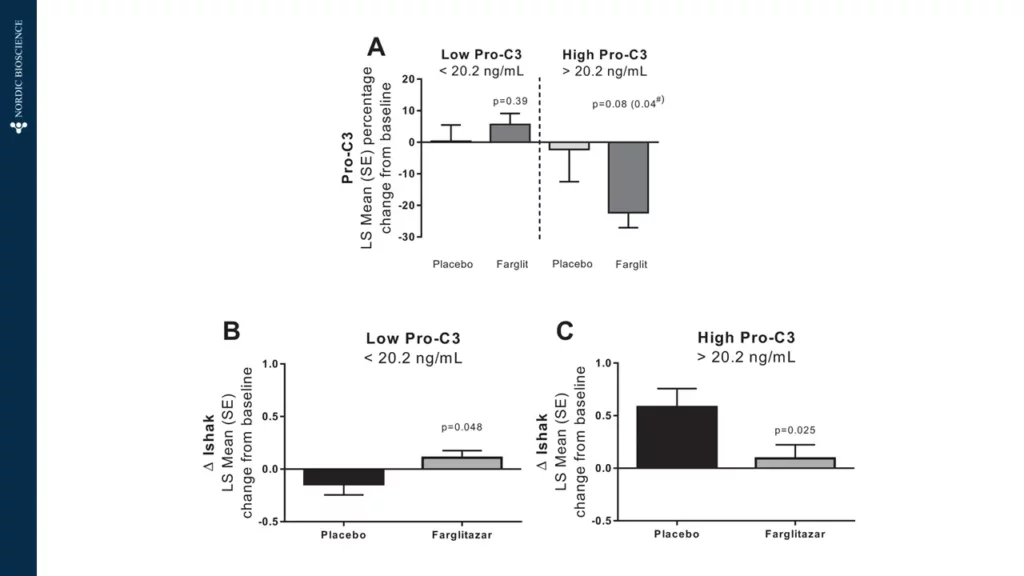
Although there are several diagnostic tools and measures of disease activity in viral liver diseases, some are invasive while others have limited predictive power. Predicting disease progression, especially in cirrhosis, has proven difficult.
Patients respond differently to viral infections, and predicting which patients will progress and when is currently impossible. There is a need for new treatments that can better control or cure viral liver disease. To achieve this, non-invasive tests (NITs) are needed to improve the screening process and the evaluation of treatment efficacy.
Did you know?
The use of non-invasive biomarkers in viral liver diseases can accelerate the drug development process and enable personalized treatment options for the patients at risk of progression to cirrhosis, therefore, preventing end-stage liver disease death.