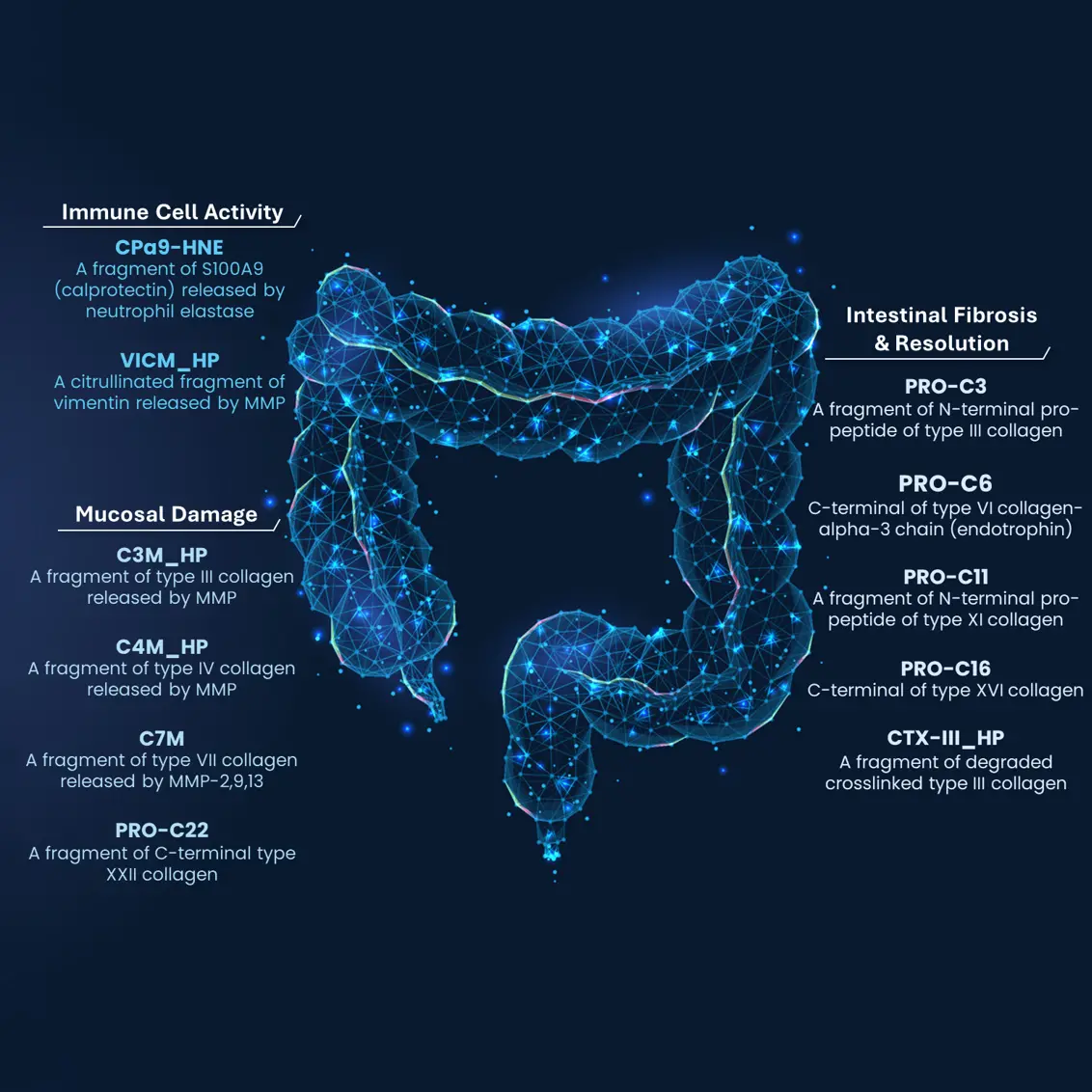
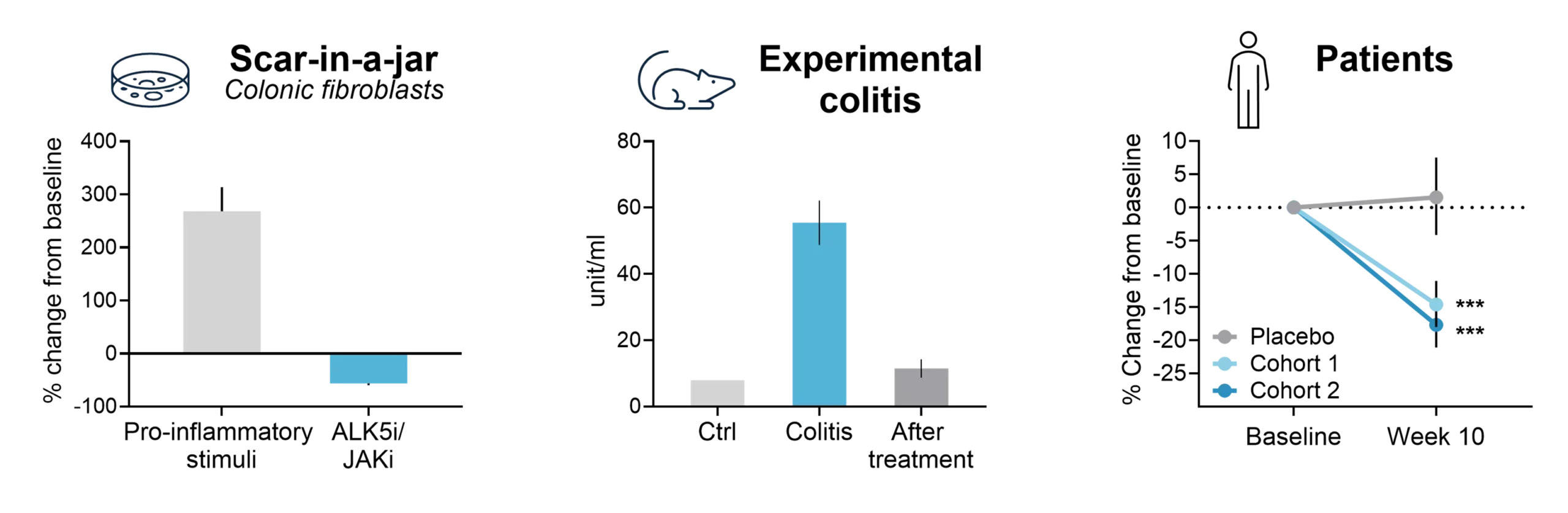Crohn’s disease is characterized by chronic inflammation leading to progressive mucosal destruction and scar tissue formation through remodeling of the extracellular matrix (ECM).
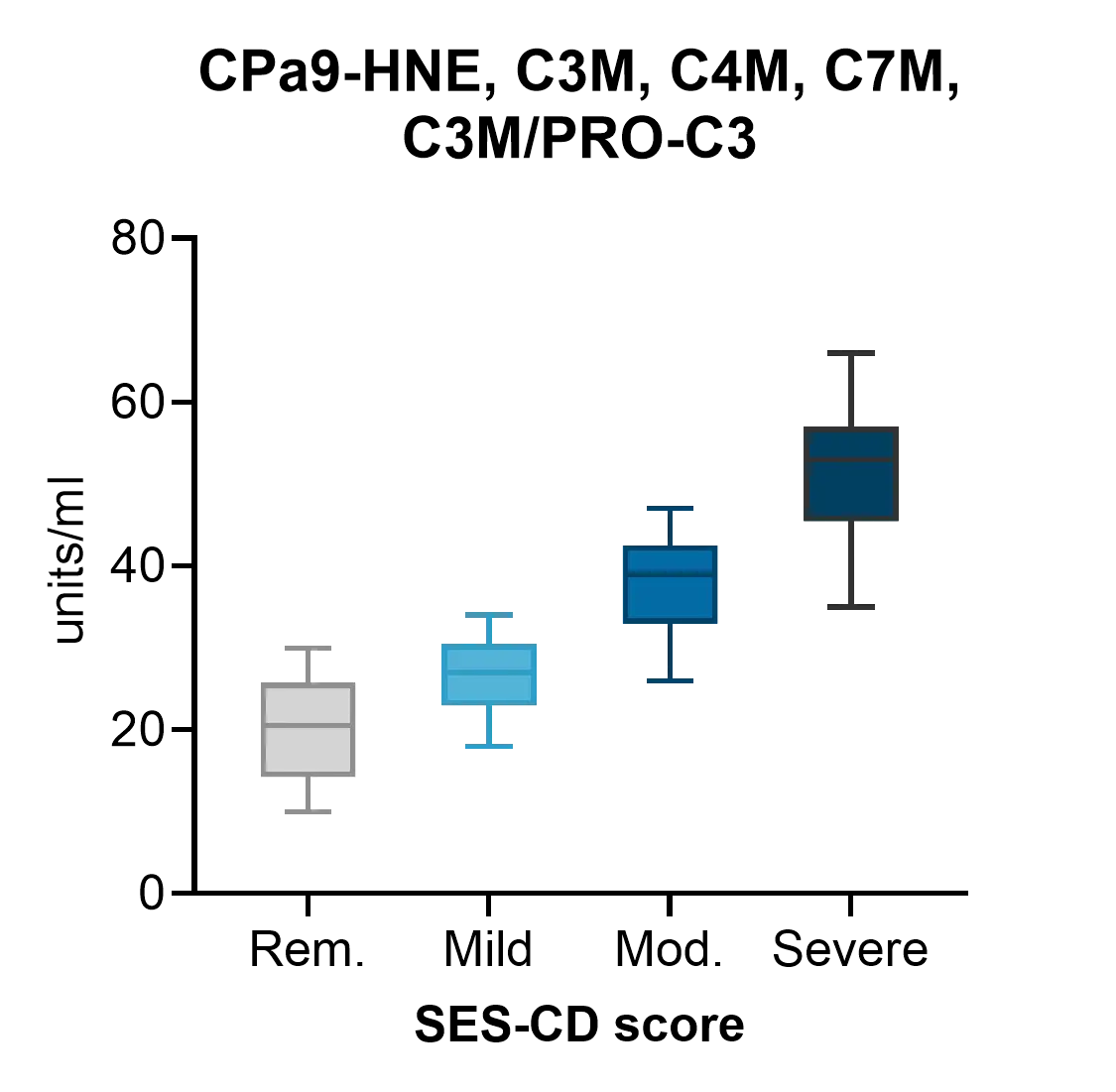
The Nordic IBDTrace™ biomarker panel quantifies the degree of inflammation (nordicCPa9-HNE™, VICM), mucosal damage (e.g., C3M, C4M, C7M), and fibrotic processes (e.g., nordicPRO-C3™, nordicCTX-III™, nordicPRO-C6™) by measuring fragments of protease-mediated degradation of mucosal proteins released into the bloodstream.
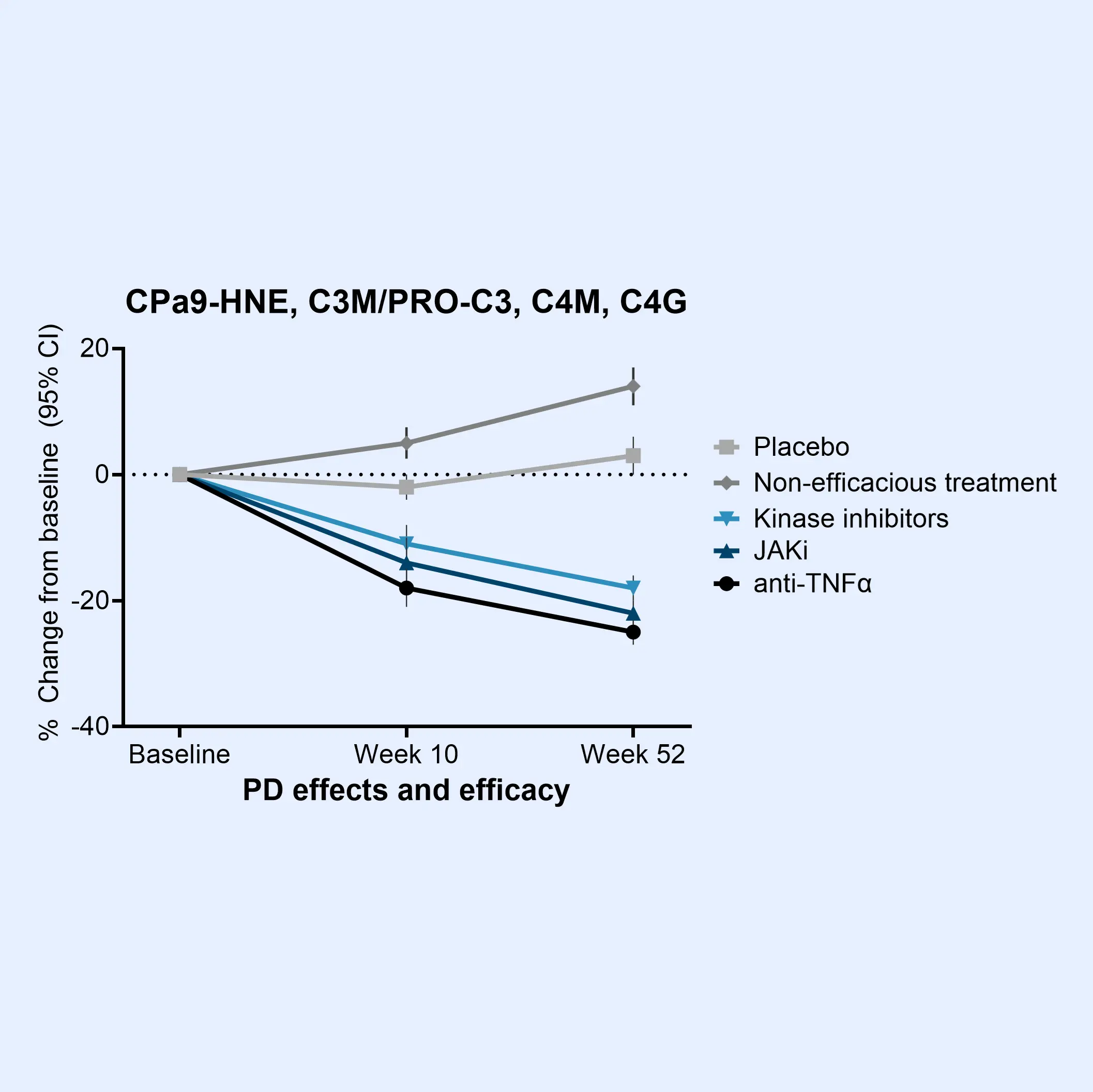
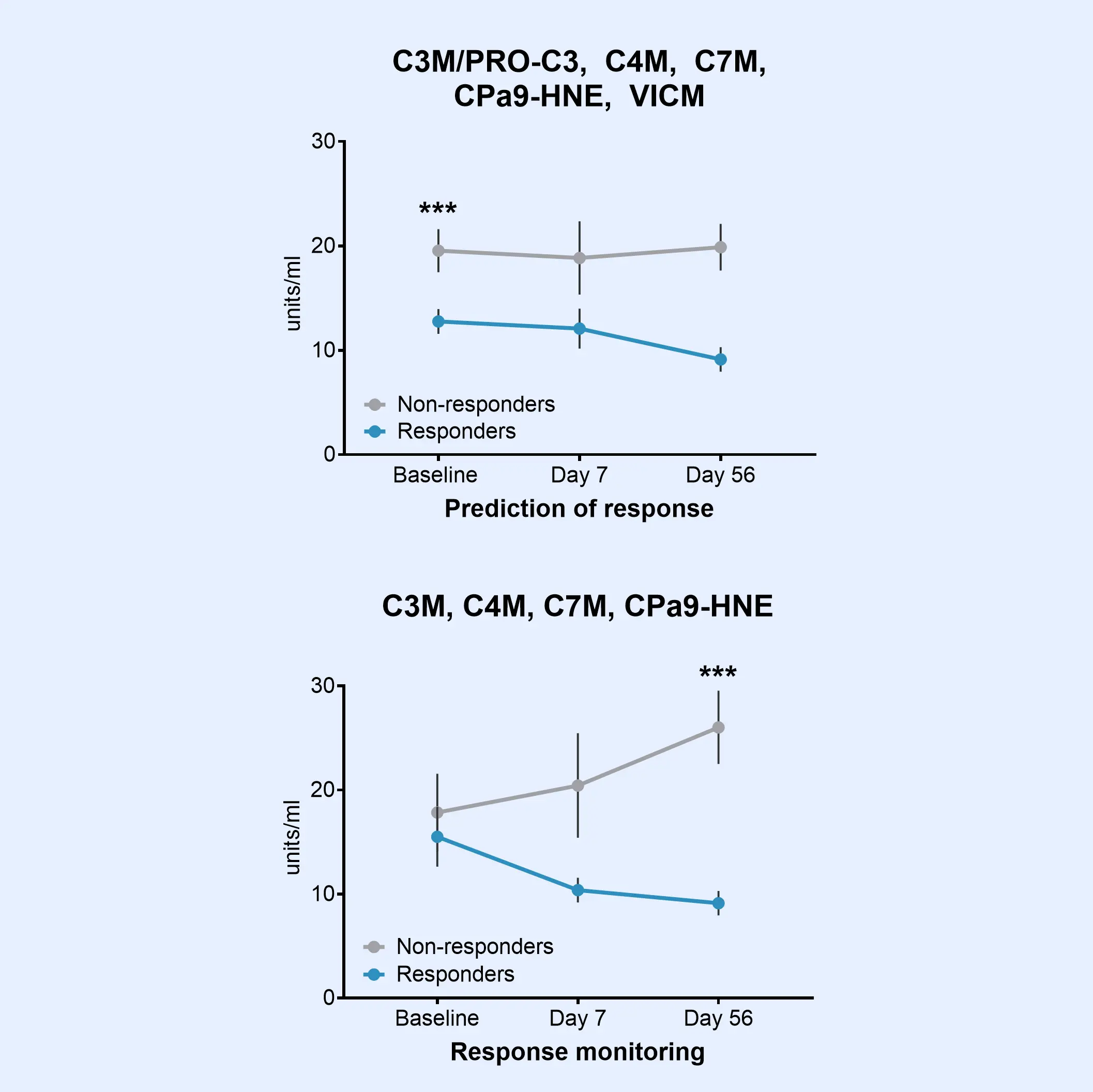
The Nordic IBDTrace™ biomarker panel provides a blood-based approach to refine drug development strategies and accelerate the path to effective treatments. By offering mechanistic insights into ECM remodeling and immune cell activity, IBDTrace™ biomarkers improve patient stratification, support internal decision-making, and enhance overall efficiency in clinical trials.
Additionally, these biomarkers can help elucidate the mode of action of investigational therapies while serving as a non-invasive alternative to frequent procedures like endoscopy.