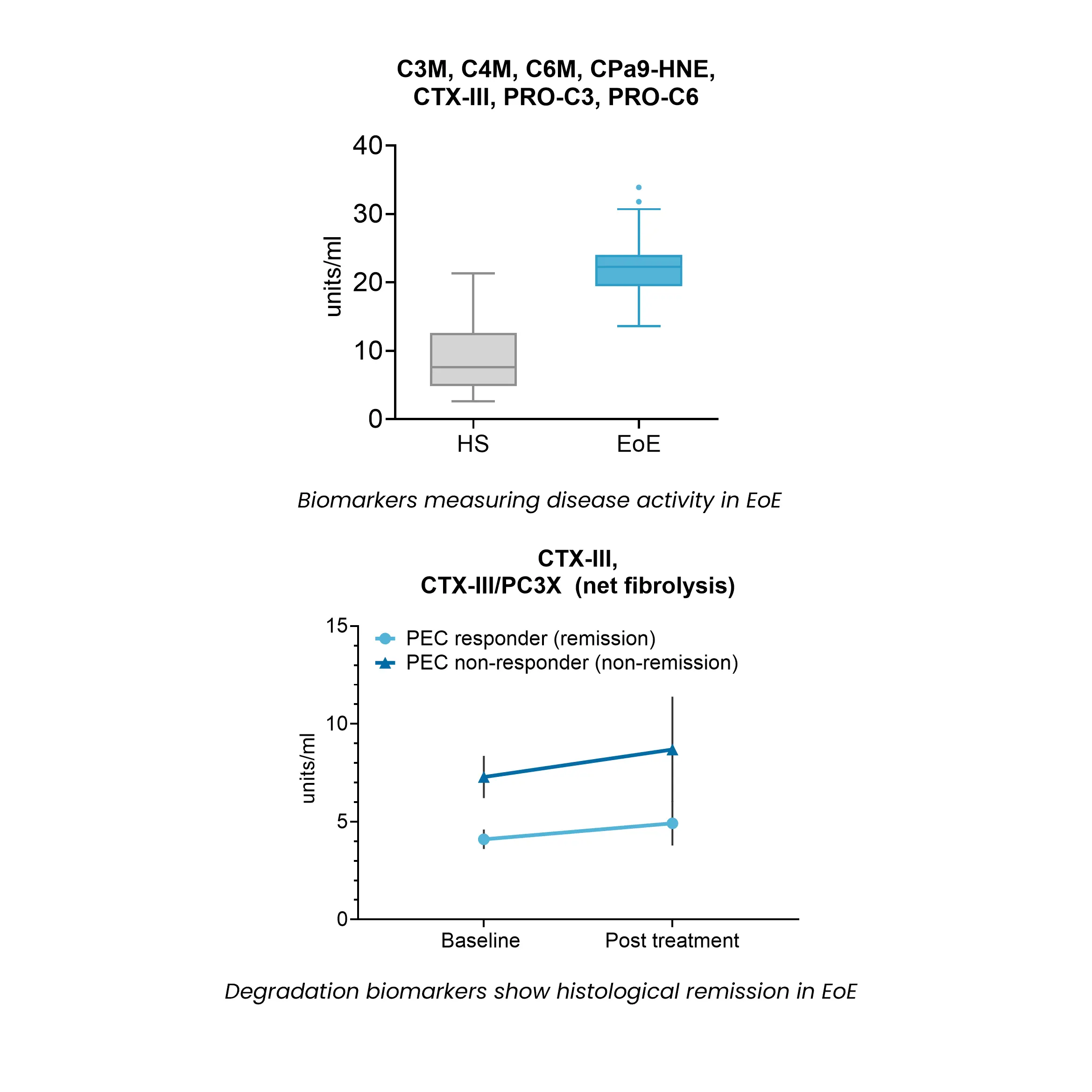
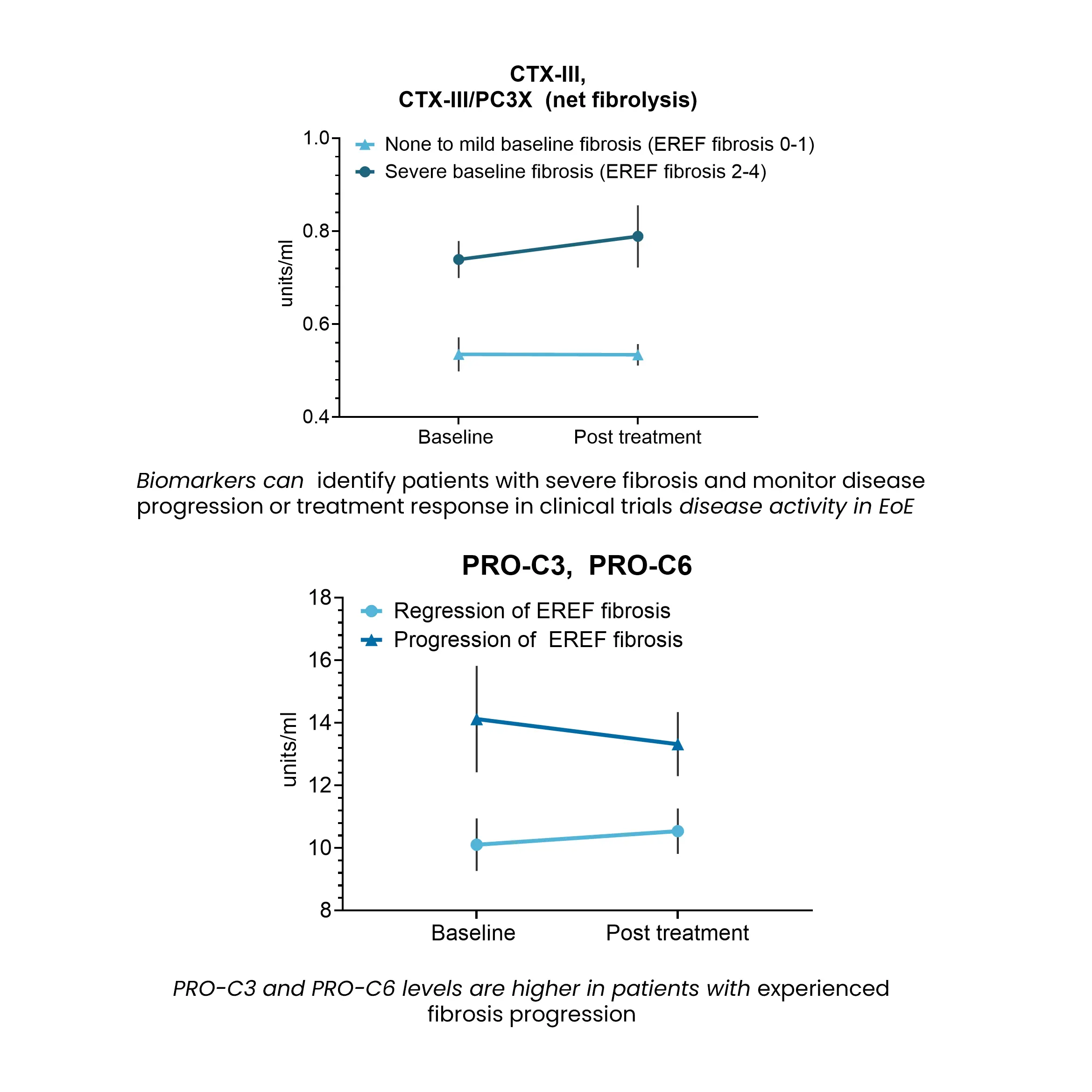
Esophageal fibrosis is characterized by excessive extracellular matrix deposition, primarily collagen accumulation within the lamina propria, leading to structural remodeling and impaired tissue function.
Biomarkers reflecting collagen turnover provide a non-invasive approach to assessing disease activity, monitoring fibrosis progression, and evaluating the impact of therapeutic interventions in EoE.
By capturing dynamic changes in extracellular matrix remodeling, these biomarkers support drug development efforts, offering insights into target engagement, treatment response, and disease modification.