Hot and Cold Fibrosis: The Role of Serum Biomarkers to Assess the Immune Mechanisms and ECM-Cell Interactions in Human Fibrosis
March 13, 2025
The novel concept of “hot and cold fibrosis”
Fibrosis develops through a complex interplay between the extracellular matrix (ECM) and the immune system. The recently emerged novel concept of “hot” and “cold” fibrosis helps understanding the immune landscape within fibrotic tissues and how ECM and immune cell interactions could be new targets for future fibrosis treatment.
In the review “Hot and Cold Fibrosis: The Role of Serum Biomarkers to assess the Immune Mechanisms and ECM-Cell Interactions in Human Fibrosis” by A. Zawadzki, D.J. Leeming, A.J. Sanyal, Q.M. Anstee, J.M. Schattenberg, S.L. Friedman, S.D. Schuppan, M.A. Karsdal, published at the Journal of Hepatology (impact factor 26.8; top 100 science journals worldwide) provides a new angle of describing fibrosis based on the presence (hot) or absence (cold) of immune cells in the fibrotic tissue and highlights the importance of interactions between the ECM, the immune system and collagens for fibrosis development and progression.
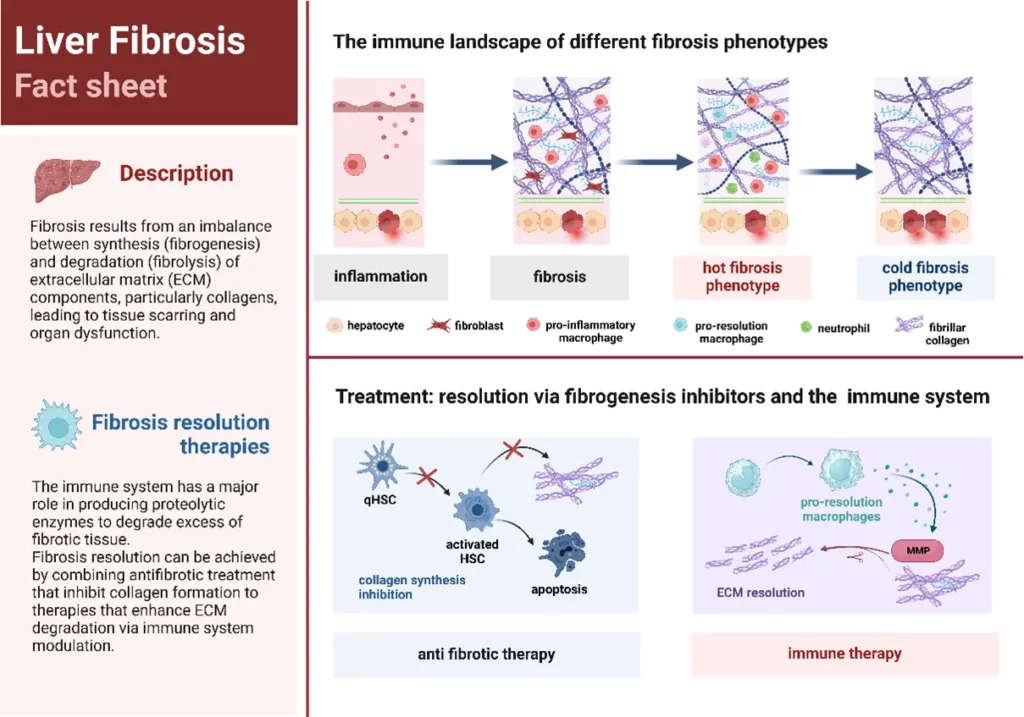
Biomarkers reflecting ECM remodeling are promising tools to monitor fibrotic and inflammatory processes that occur in hepatic diseases and therefore, allow to identify disease profiles associated either with hot or cold fibrosis.
Understanding how the interactions between ECM and immune system work and identifying distinct disease phenotypes will provide guidance for future therapy that prevents and treats fibrosis and induces its regression from two different standpoints: inhibiting fibrosis formation by intercepting fibrogenesis with anti-fibrotic drugs and/or enhancing fibrosis resolution/ECM degradation by stimulating immune cells to secrete proteolytic enzymes to break down the excess of fibrotic tissue. A typical hot fibrosis phenotype has the advantage of having suitable conditions for immune therapy approach since it has immune cells present in the fibrotic tissue that can be modulated to a pro-resolution phenotype.
Biomarkers reflecting fibroblast activity such as PRO-C3 and PRO-C6 can be useful to identify patient profiles with higher fibrogenic activity that can be treated by anti-fibrotic therapies. PRO-C3 and PRO-C6 can be dynamically modulated by anti-fibrotic drugs and allow to investigate drug response and pharmacodynamics of new anti-fibrotic therapies.
Biomarkers reflecting immune cells activity, including VICM (macrophage activity), CPa9-HNE (neutrophil activity) and C4G (T cell activity) can help to identify a patient profile associated to a hot fibrosis phenotype which would benefit by immune therapy. In addition, biomarkers reflecting ECM degradation (fibrolysis) such as CTX-III allow to monitor the efficiency of such immune therapies to stimulate the immune cells to degrade the excess of fibrotic tissue.
Combining an anti-fibrotic drugs to therapy that stimulate immune cells can be monitored by biomarkers that reflect different biological processes and may help to investigate treatment efficacy.




