- The cancer field needs better actionable biomarkers to support drug development and identify patients responding to treatment.
- The commonly observable lack of response to treatment can be attributed to tumor fibrosis.
- Tumor fibrosis biomarkers should therefore be implemented in all clinical cancer trials.
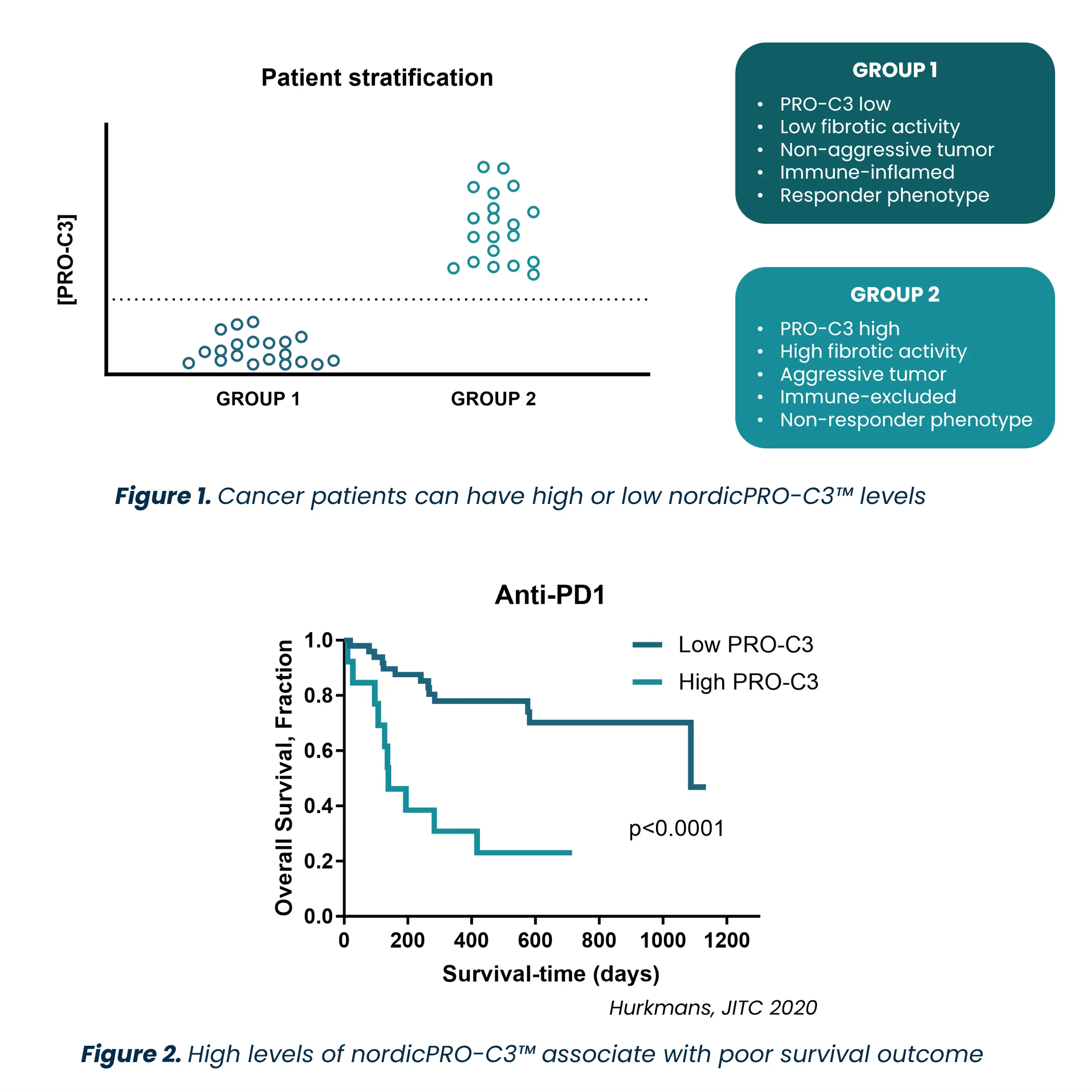
- NordicPRO-C3™ (formerly PRO-C3) is a liquid biopsy biomarker that measures fibrotic activity in patients with solid tumors.[1]
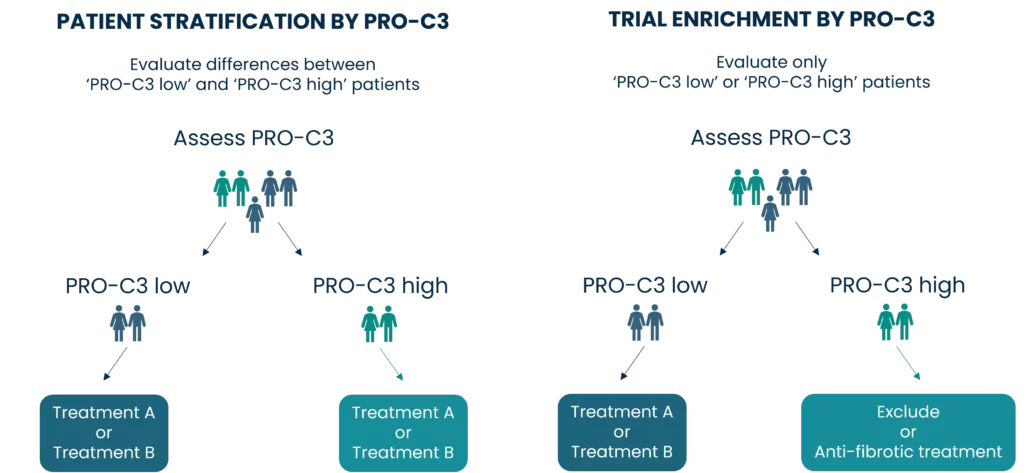
- The US FDA supports the use of nordicPRO-C3™ as a non-invasive biomarker for clinical trial enrichment and patient stratification across all solid tumors.
NordicPRO-C3™ in Tumor Fibrosis
A liquid biopsy enabling the identification of cancer patients for clinical trial enrichment and patient stratification.