Pliant Therapeutics Publishes Positive Data From Their INTEGRIS-IPF Phase 2a Trial of Bexotegrast Featuring PRO-C3 Biomarker
January 24, 2023
Pliant Therapeutics publishes positive data from their INTEGRIS-IPF Phase 2a trial of bexotegrast.
Pliant met the primary, secondary and exploratory endpoints and demonstrated good tolerability and a significant increase in forced vital capacity (FVC) in patients with IPF. An increase in FVC over 12 weeks is remarkable and bodes well for future therapeutic options for IPF patients.
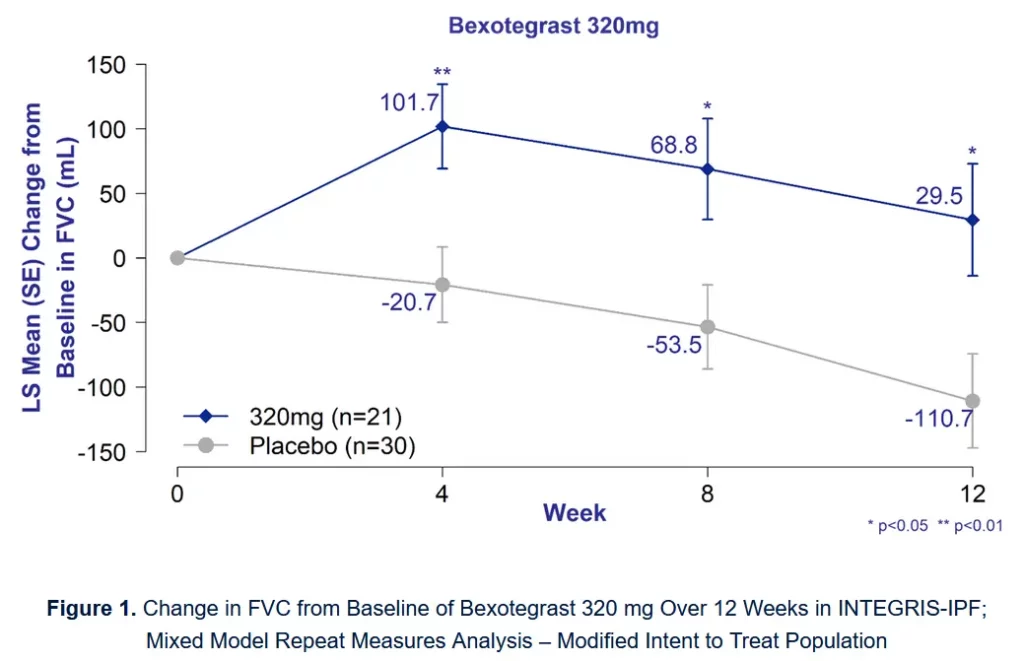
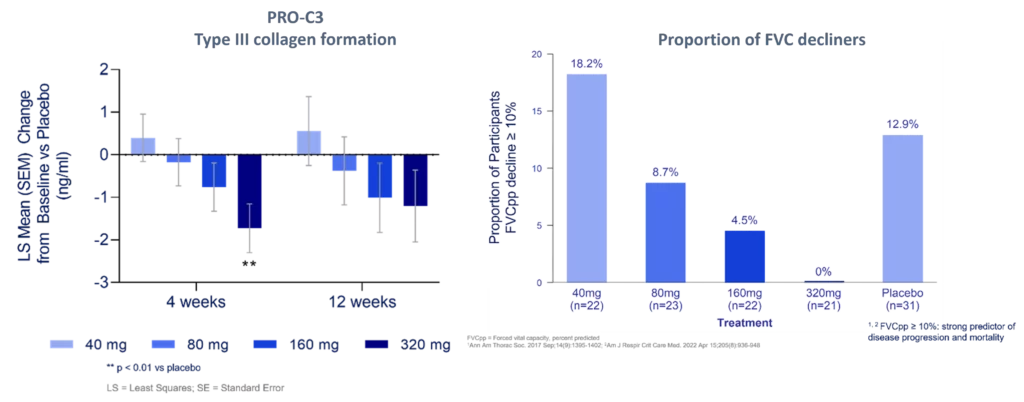
Our fibrogenesis biomarker PRO-C3 was dose-dependently decreased by bexotegrast at 4 and 12 weeks, supporting that the therapy had antifibrotic effects. The effects on PRO-C3 were accompanied by comparable effects on the proportion of progressors in each arm. At the same time, reductions were observed in quantitative lung fibrosis, further supporting the positive effects of bexotegrast on IPF.
Nordic Bioscience is pleased to have supported this trial and is eager to see the results of the 24-week treatment, as well as further progress in the Phase 2btrial.




