Quantification of active TGFβ in serum predicts treatment response in solid tumors
December 6, 2024
TGFβ signaling: a game changer in personalized cancer treatments?
Treatment outcomes are deeply influenced by the tumor microenvironment (TME) where TGFβ (Transforming Growth Factor Beta) has emerged as a critical factor that can dictate the success—or failure—of therapeutic strategies.
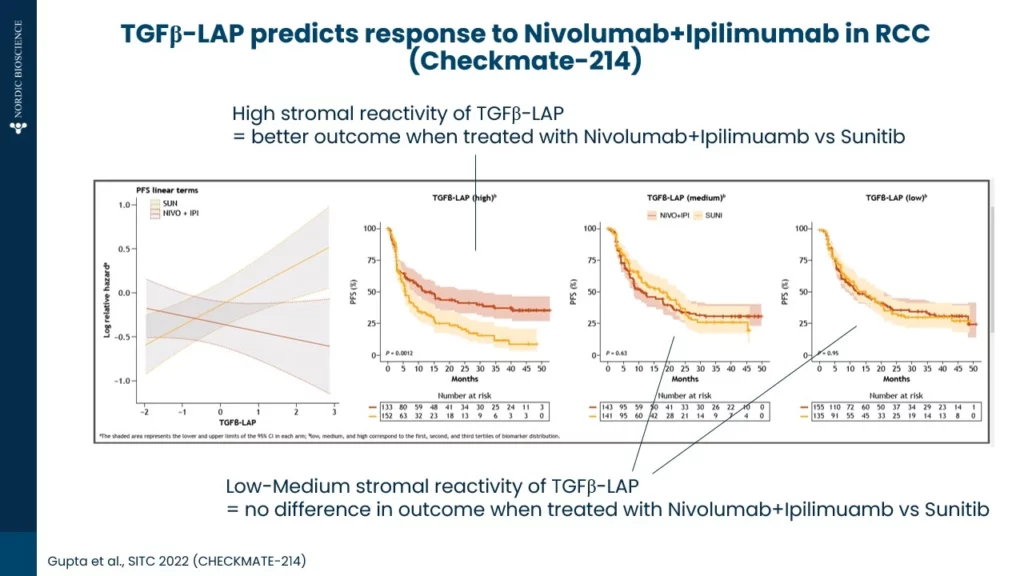
As shown below (Figure 1.) in an example from renal cell carcinoma (RCC), high TGFβ activity contributes to poor responses to tyrosine kinase inhibitors (TKIs) as compared immune checkpoint inhibitors. TGFβ activity was measured by the TGFβ-LAP biomarker in pre-treatment serum.
Conventional TGFβ assays are affected by platelets releasing TGFβ during clotting (drastically affecting the measurements in serum). In contrast, the TGFβ-LAP biomarker quantifies a cleaved latent activating protein (LAP) fragment which is independent of platelet TGFβ release providing a novel tool to indirectly measure TGFβ in serum.
Why does this happen?
TKIs, such as Sunitinib, are primarily targeting angiogenesis and signaling pathways critical for tumor growth. However, elevated TGFβ promotes an immunosuppressive and fibrotic TME, counteracting these therapies by enhancing tumor resilience and protecting vasculature from the intended effects of TKIs. On the other hand, anti-PD-1/anti-CTLA-4 immuno-oncology (IO) therapies such as Nivolumab and Ipilimumab, which aim to re-activate T-cell responses, may be relatively more effective even in a TGFβ-rich environment.
Personalizing treatment (TKI vs. IO) with biomarker-driven approaches
Understanding TGFβ activity could be a game-changer in personalizing cancer treatments. Biomarkers that measure TGFβ levels or its downstream effects might help clinicians choose between TKIs or immunotherapy – or even better, design combination regimens. This underscores the importance of a biomarker-driven approach to cancer treatments. As we continue to unravel these mechanisms, the goal remains clear: to turn the TME from a shield into a target.
Our approach is designed to be implemented into clinical trials and research programs on TKI vs. IO, and to help explore the intersection of TGFβ biology and therapeutic resistance.




