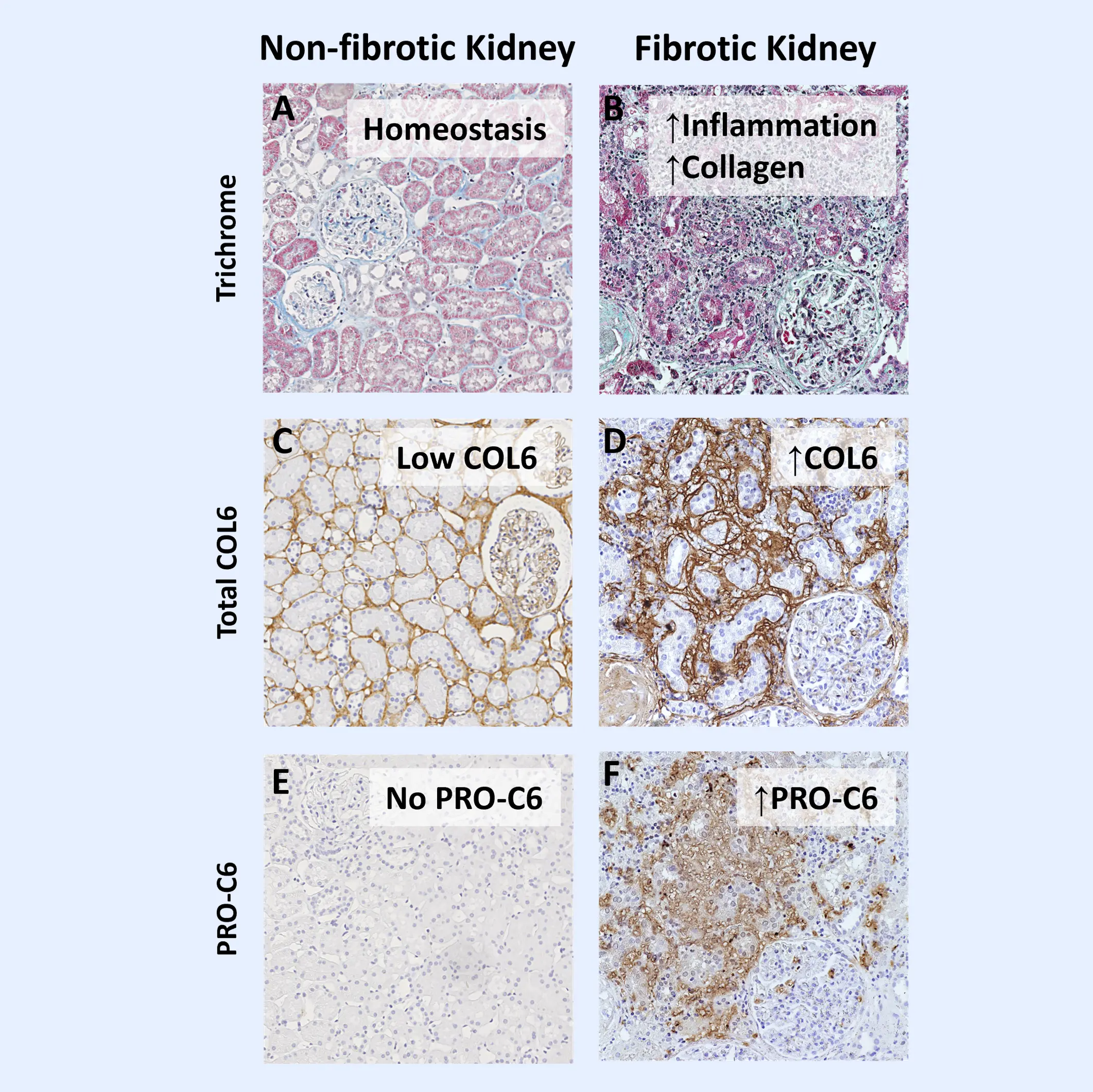
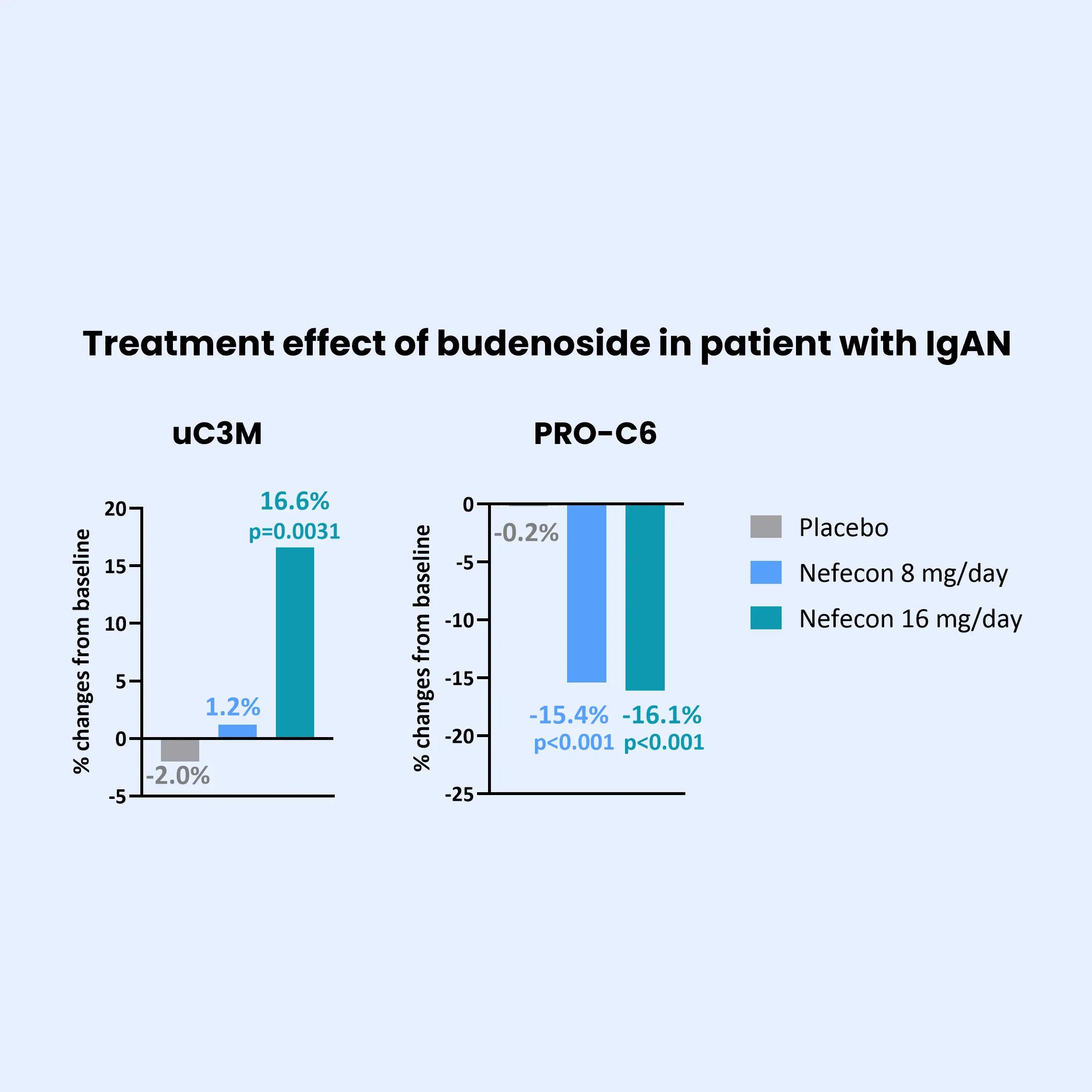
IgA nephropathy (IgAN) is the most common form of glomerulonephritis worldwide and a leading cause of end-stage kidney disease (ESKD). IgAN is characterized by the deposition of IgA in the glomerular basement membrane. While the clinical trajectory of IgAN varies among individuals, its progression is frequently driven by subclinical and persistent kidney fibrosis. Excessive accumulation of extracellular matrix (ECM) proteins occurs during fibrosis, which disrupts cellular organization and function within the kidney.
Current clinical markers are limited in their ability to detect early fibrotic changes or to monitor dynamic treatment responses. Biomarkers offer a powerful tool to uncover dynamic disease processes and enable earlier, more informed decision-making. Biomarkers of the ECM can provide direct insight into active fibrogenesis—the key mechanism underlying irreversible kidney damage.