NordicPRO-C6™ (Kit in a Box)
PopularA fragment of the C-terminal end of type VIa3 collagen measuring the Endotrophin signaling molecule, enabling non-invasive quantification of fibrogenesis, tissue remodeling and wound healing across chronic diseases.
Manual ELISA kit version: The kit is FOR RESEARCH USE ONLY (RUO) and can only be used in NON-CLINICAL settings. It can not be used IN DIAGNOSTIC PROCEDURES. Available in both human and rodent versions.
What is a Kit in a Box product?
Key features and values
- Measures a specific fragment of type VI collagen produced during collagen formation.
- Reflects fibroblast activity and extracellular matrix remodeling in multiple organ systems.
- Enables non-invasive assessment of fibrotic progression in cardiovascular, kidney, lung, intestinal and metabolic diseases and is a risk biomarker across a plethora of chronic diseases.
- Facilitates risk stratification and prognosis in patients with chronic and progressive diseases.
- FDA-supported use in HFpEF trials for patient enrichment.
- Monitors treatment response and confirms mechanism of action in clinical trials.
- Applicable for research into the mechanisms and dynamics of fibrosis and tissue repair.
- Complements other biomarkers to provide a comprehensive profile of collagen turnover and fibro-inflammatory activity.
Description
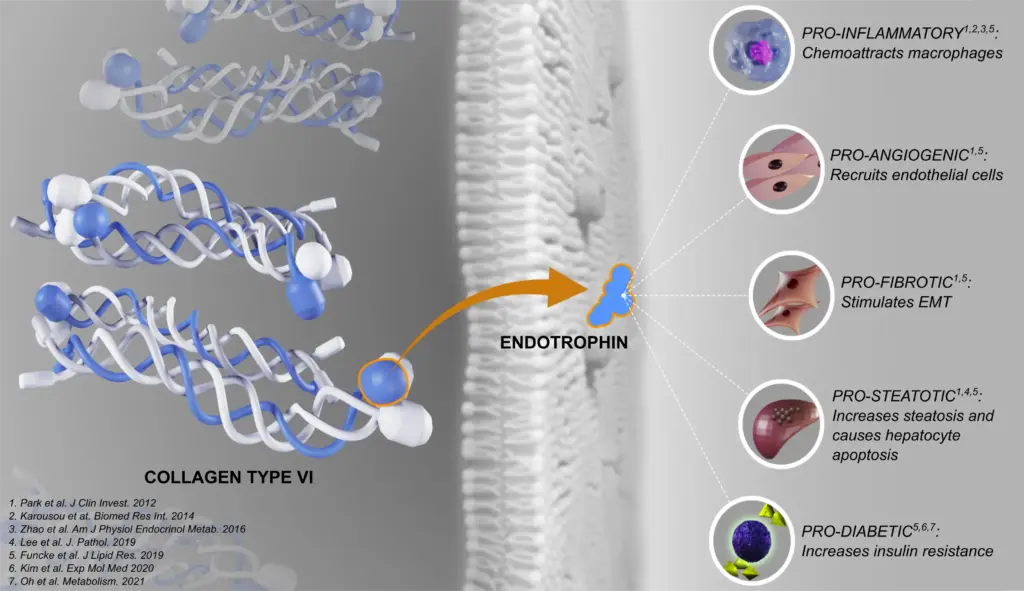
The nordicPRO-C6™ biomarker assay quantifies a fragment of type VI collagen generated during collagen formation, serving as a specific marker of active fibrogenesis and extracellular matrix remodeling. Moreover, it targets a type VI collagen-derived bioactive molecule called endotrophin, which has proven pro-fibrotic and pro-inflammatory signaling properties.
This non-invasive assay provides valuable insight into fibrotic processes underlying a wide range of chronic diseases, including those affecting the heart, kidney, lung, intestinal and metabolic systems. By capturing dynamic changes in type VI collagen synthesis, and the release of the dangerous signaling molecule Endotrophin, nordicPRO-C6™ supports disease monitoring, risk assessment, and therapeutic evaluation in clinical and research settings. Used alongside other collagen biomarkers, nordicPRO-C6™ enables a comprehensive assessment of collagen turnover and fibro-inflammatory progression, informing precision-medicine strategies in anti-fibrotic therapy and patient management.
At Nordic Bioscience, our approach to measurement is rooted in decades of innovation and a deep understanding of fibrosis. As the originators of nordicPRO‑C6™, we perfected the biomarker over 15+ years of clinical trials, built on more than 30 years of expertise in extracellular matrix biomarkers. Today, nordicPRO‑C6™ is a household name known to ensure you receive data that is both precise and actionable.
As a prognostic biomarker, nordicPRO-C6™ supports patient selection by identifying individuals more likely to experience disease progression or clinical outcomes — helping enrich trial populations, boost statistical power, and increase the likelihood of success.
NordicPRO-C6™ has demonstrated value in cardiovascular, kidney, lung, and metabolic diseases, predicting disease progression and clinical outcomes (figure outcomes). In Crohn’s disease (CD), patients can be differentiated into endotypes, with elevated levels observed in those with fibrostenotic strictures compared to those with luminal disease.
NordicPRO-C6™ has received a letter of support from the FDA encouraging its use to select HFpEF patients with high disease activity for enrollment in clinical trials.
As a pharmacodynamic marker, NordicPRO-C6™ provides a powerful tool for monitoring the efficacy of treatments that lower the risk of an outcome.
NordicPRO-C6™ provides a powerful tool for monitoring treatment efficacy and tracking disease progression over time. Furthermore, it serves as a critical decision-making tool in clinical trials, helping researchers assess treatment impact, understand the mechanism of action, and accelerate drug development.
We focus on your research needs
- Our nordicPRO‑C6™ assay is proven and validated over dozens of clinical trials and is trusted by leading global pharmaceutical partners.
- Operated in a CAP/CLIA‑certified, ISO‑compliant laboratory, our methods provide a robust foundation for clinical decision‑making, offering clinical and regulatory excellence.
- With a rapid 24-hour turnaround and an optimized sample requirement of just 60 µL, we provide operational efficiency. Our workflow is designed to support timely and efficient research.
- Our exclusive ProteinFingerPrint Technology™ turns data into insight. We transform complex collagen turnover data into clear insights, empowering you to improve patient selection, monitor treatment efficacy, and drive precision in fibrosis research.
Patient selection for more efficient clinical trials
Clinical trials are a critical and costly step in drug development. Reducing trial size while maintaining robust data is essential for accelerating market entry and managing costs.
NordicPRO‑C6™ transforms clinical trial efficiency by enabling precise pre-stratification of patients. By identifying individuals with active fibroblasts who are most likely to respond to treatment, nordicPRO‑C6™ helps optimize patient selection, ensuring trials enroll the right participants from the start. This not only enhances trial success rates but also significantly reduces the number of enrollees needed—saving time, resources, and costs while increasing confidence in the therapeutic’s efficacy.
By integrating nordicPRO‑C6™ into clinical trial design, drug developers can streamline recruitment, improve study outcomes, and bring innovative fibrosis-targeted therapies to market faster.
Monitor drug response
Not all patients respond equally to treatment—many experience varying levels of efficacy and safety, while some fail to respond at all. This leads to wasted resources, ineffective care, and increased healthcare costs.
NordicPRO C6™ provides valuable insights into MOA by detecting active fibroblasts, a key process in disease progression and treatment response. By integrating nordicPRO C6™ into clinical trials, researchers gain a deeper understanding of how a drug interacts with fibrotic pathways, supporting data-driven decisions on safety, efficacy, and patient stratification.
Effective drug development requires real-time insights into treatment efficacy and pharmacodynamic effects to ensure therapies are working as intended. Traditional endpoints often fail to capture early dynamic changes in fibrosis, delaying crucial adjustments in clinical strategies.
NordicPRO C6™ changes this by providing a powerful tool to predict treatment response in fibrotic diseases. By measuring active fibroblasts, nordicPRO C6™ helps identify patients who are most likely to benefit from specific therapies.
Nordic Bioscience’s assays and services are research use only products and services and do not qualify for medical or diagnostic purposes.
Kit in a Box – Frequently Asked Questions (FAQ)
-
It is a standard competitive ELISA using 96-well plates pre-coated with streptavidin, requiring no additional blocking.
-
Timer
Disposable containers (for dilutions)
Plate sealing tape
Calibrated micro-pipettes and tips
Mixing plates or Eppendorf tubes
Deionized or distilled water
Microplate shaker (300 ± 50 rpm)
Microplate reader (450 nm wavelength with 650 nm reference)
Vortex mixer or equivalent
Incubator (4-6°C)
Freezer (≤ -18°C)
-
The kit is suitable for:
Human cell culture medium
Human biological fluids (e.g., serum, EDTA plasma, citrate plasma)
In vitro cell-culture supernatants (e.g., fibroblast cultures)
Ex vivo model matrices (e.g., conditioned medium from precision-cut tissue slice cultures)
NordicPRO-C6™ Rodent (rat, mouse) serum version is also available. Please specify when ordering.
-
No. It is for Research Use Only in preclinical settings and not suitable for diagnostic purposes.
-
The kit remains stable for 3 months from the date of delivery.
-
Each 96-well plate accommodates approximately 80 single measurements or 40 duplicate samples. The minimum purchase requirement is two kits, enabling up to 160 single or 80 duplicate measurements.
-
The assay includes three incubation steps: an initial 30 minutes, an overnight incubation (~20 hours), and a final step of 15 minutes.
-
Yes, absorbance measurement is necessary at 450 nm with a reference at 650 nm.




