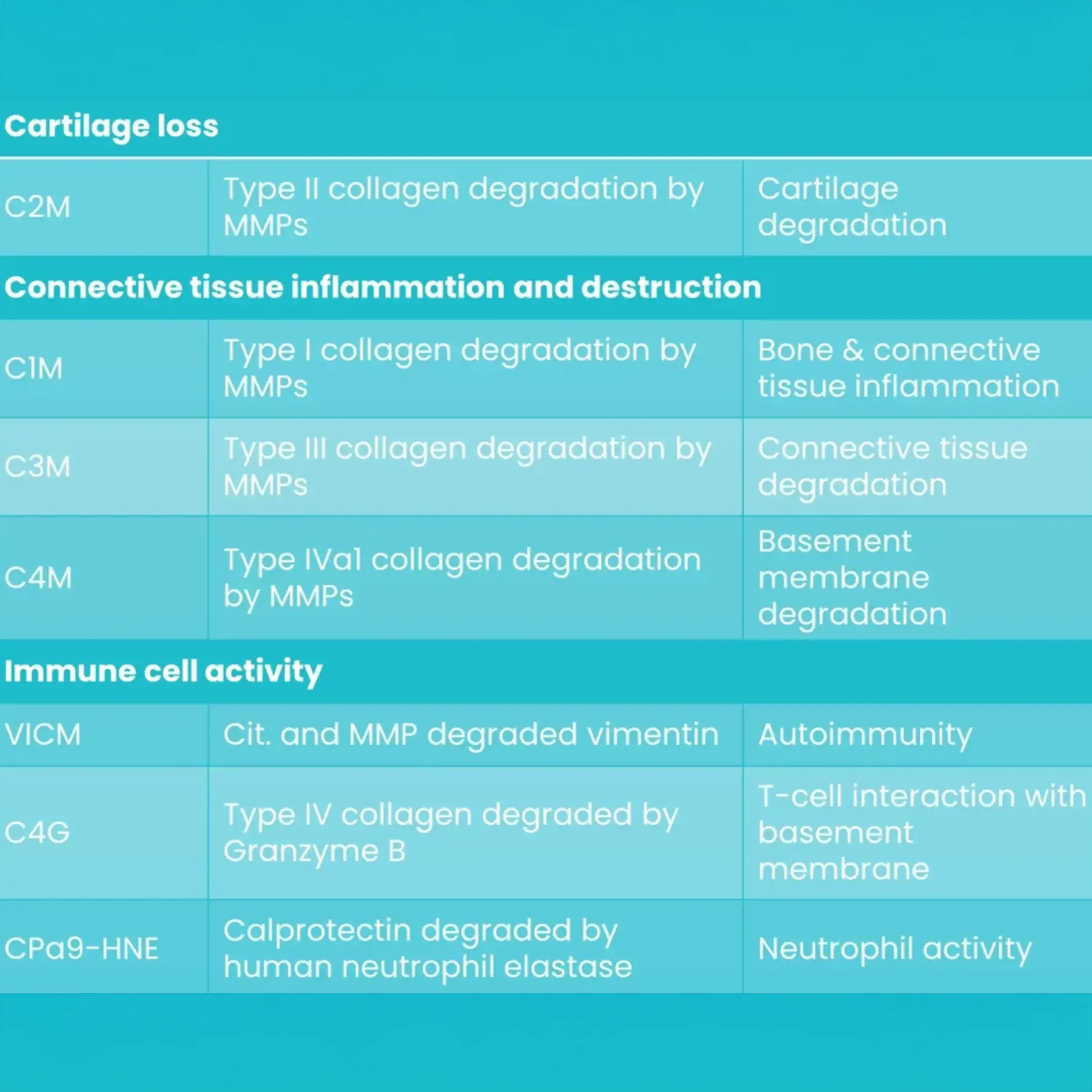
Introducing Nordic RheumaTrace™: a breakthrough biomarker panel for rheumatic diseases. Backed by three decades of research and over 200 peer-reviewed publications, this panel features seven carefully selected biomarkers.
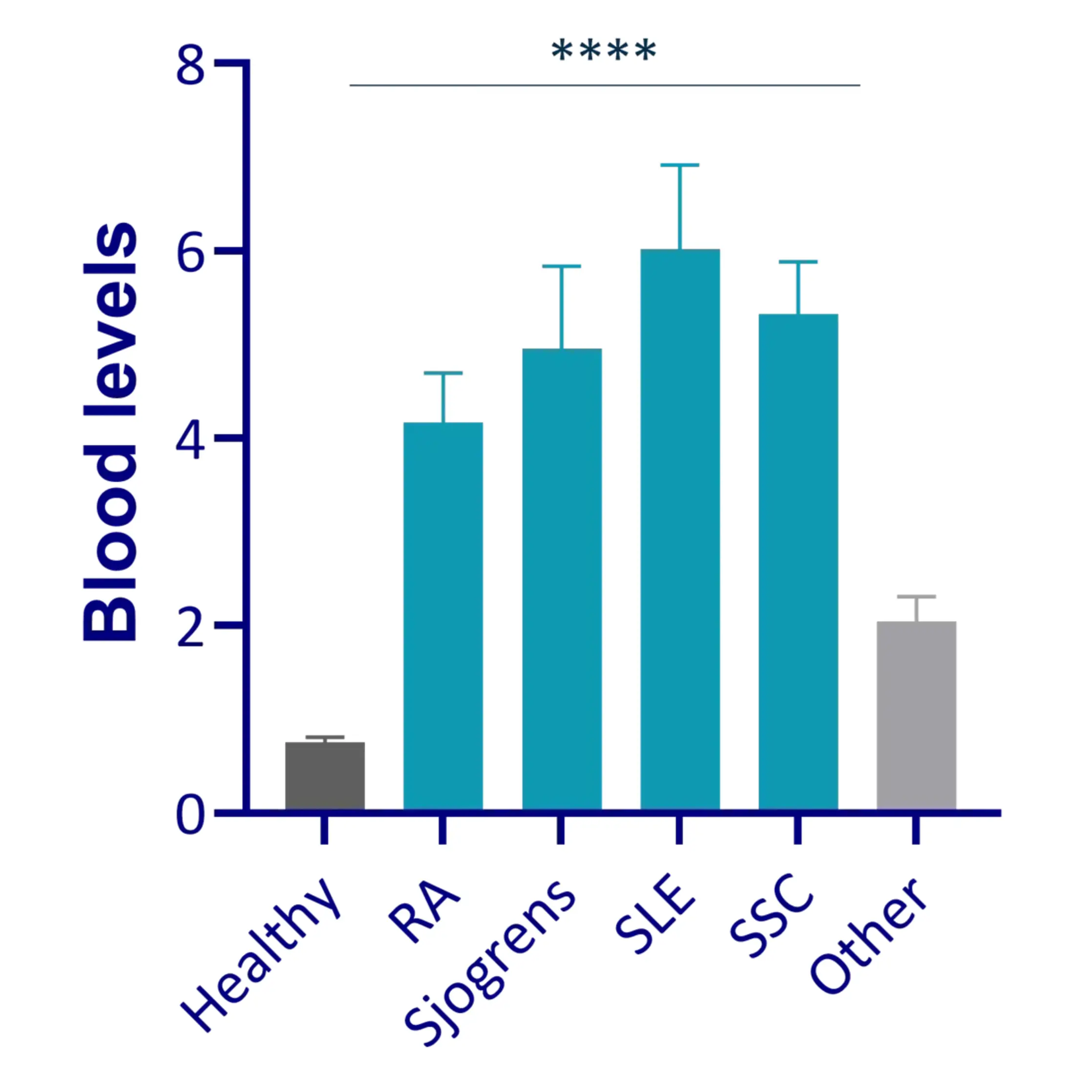
These biomarkers delve into the complexities of connective tissue degradation and immune cell dynamics, providing invaluable insights for researchers and drug developers.
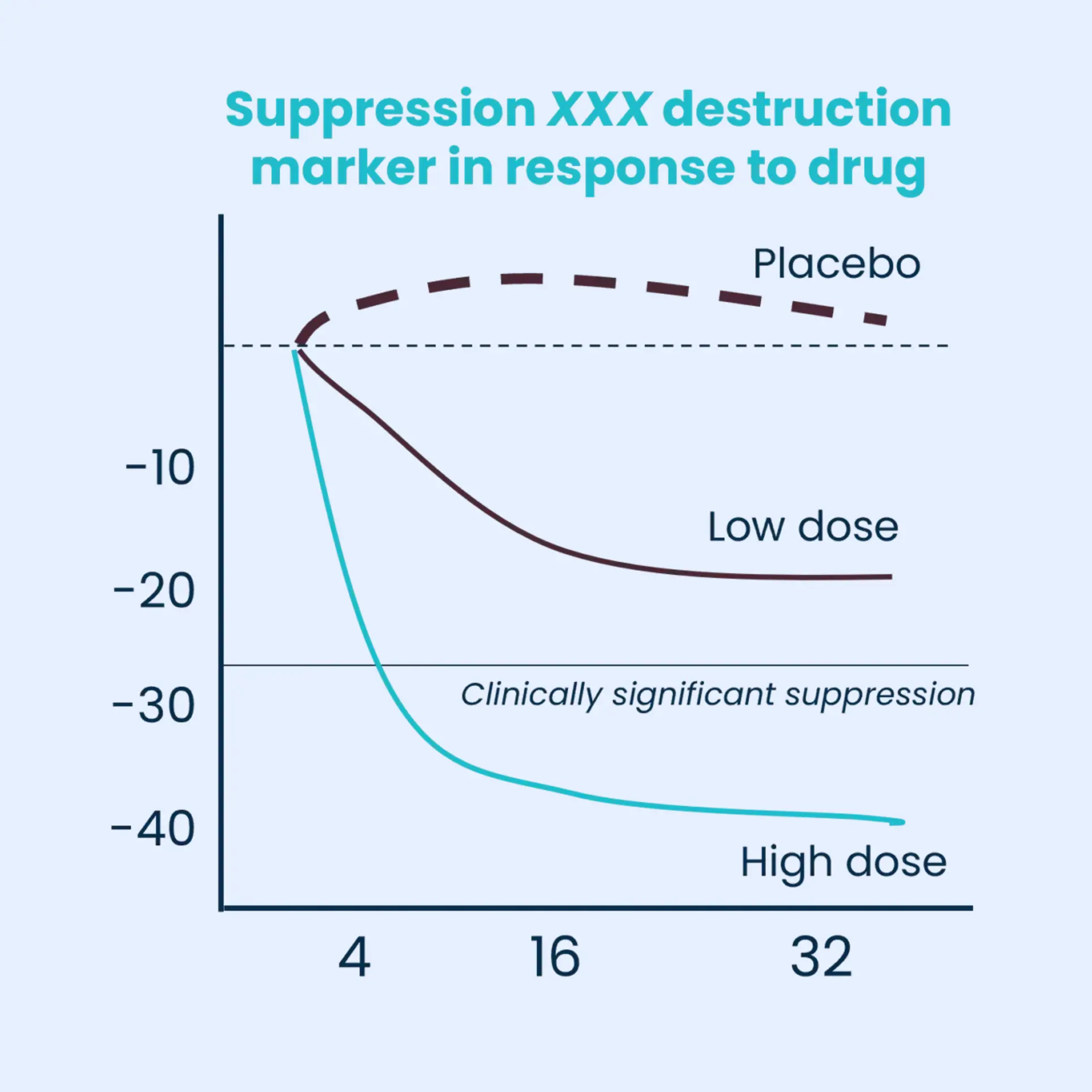
By pinpointing the disease pathway and determining the optimal dosage for positive outcomes, our panel is at the forefront of precision medicine in rheumatology.