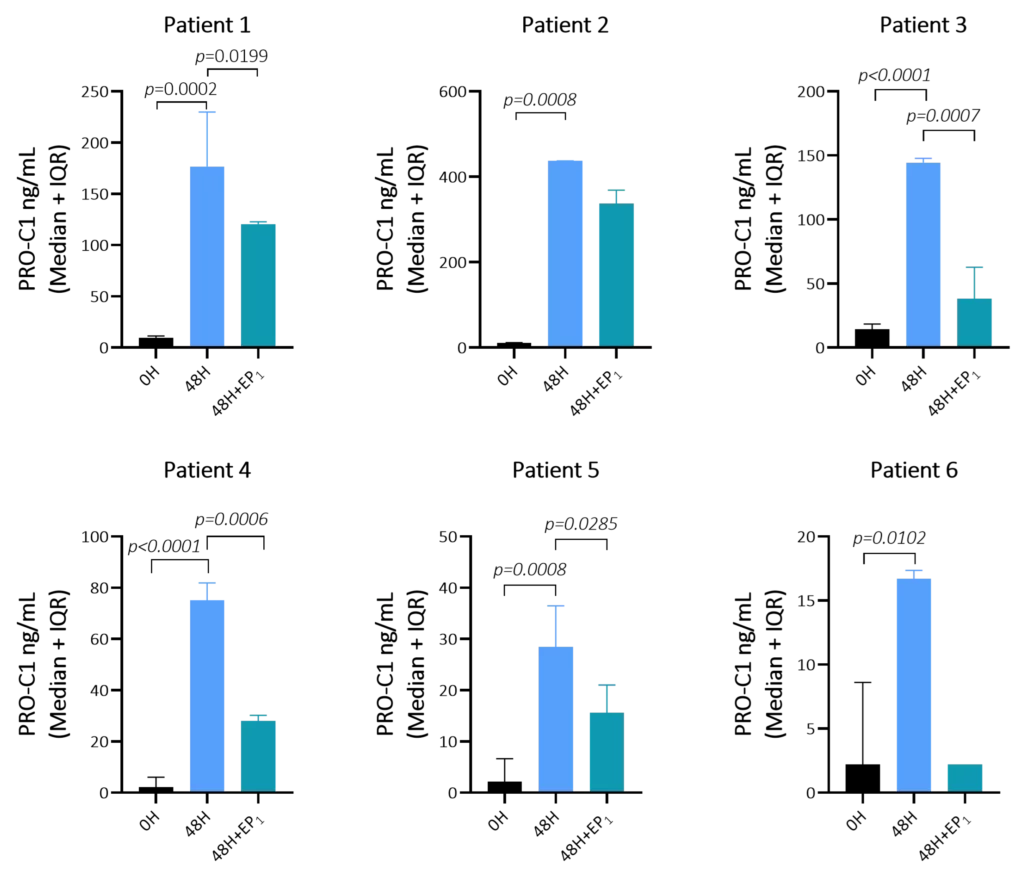Successful drug development is essential but often challenging and uncertain. To improve outcomes, it’s important to use strategies that increase predictability and lower risk. Regulatory agencies such as the FDA and EMA recognize the value of biomarker-driven approaches, especially when combined with robust translational research, to guide decision-making and accelerate progress through the pipeline.
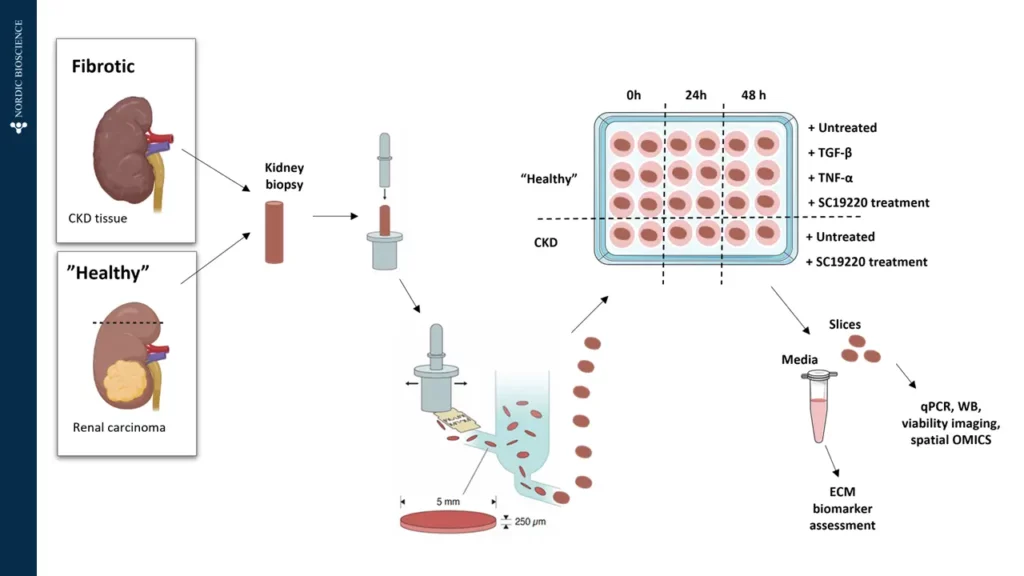
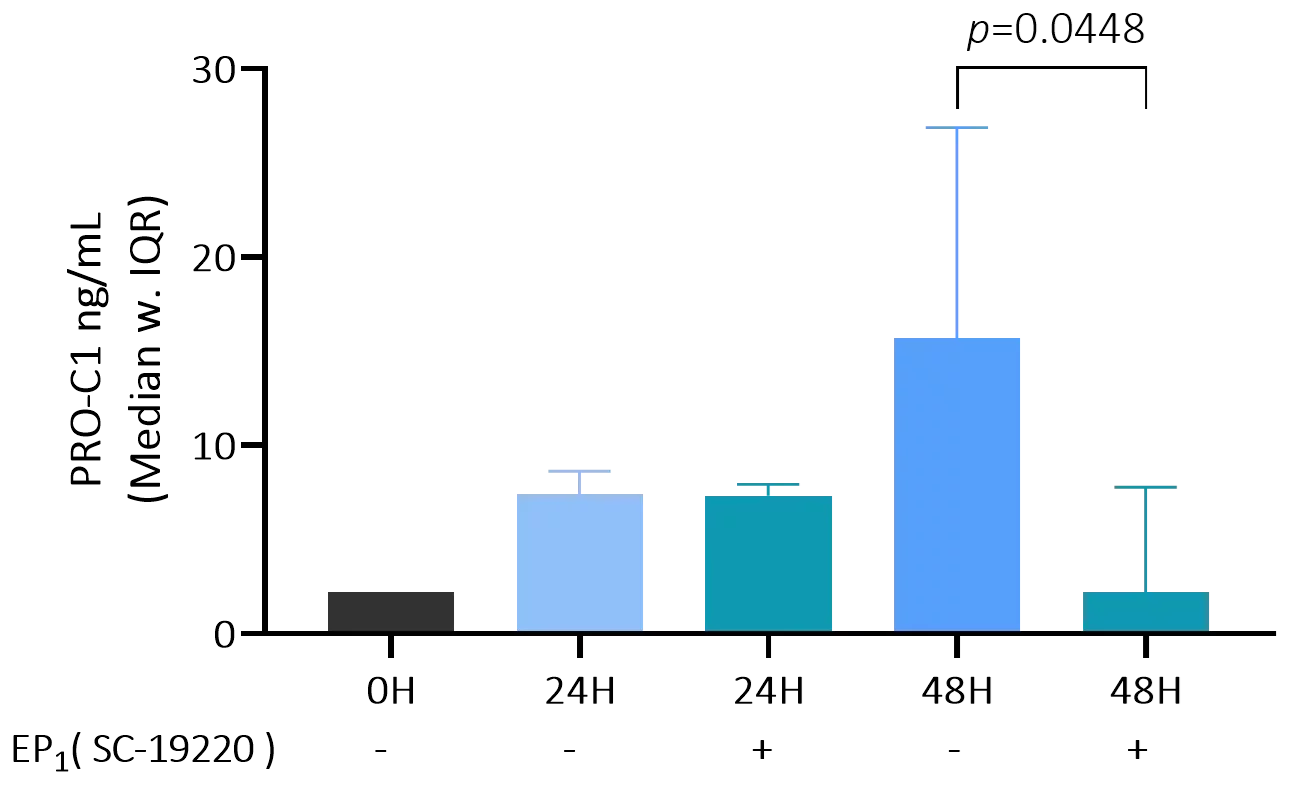
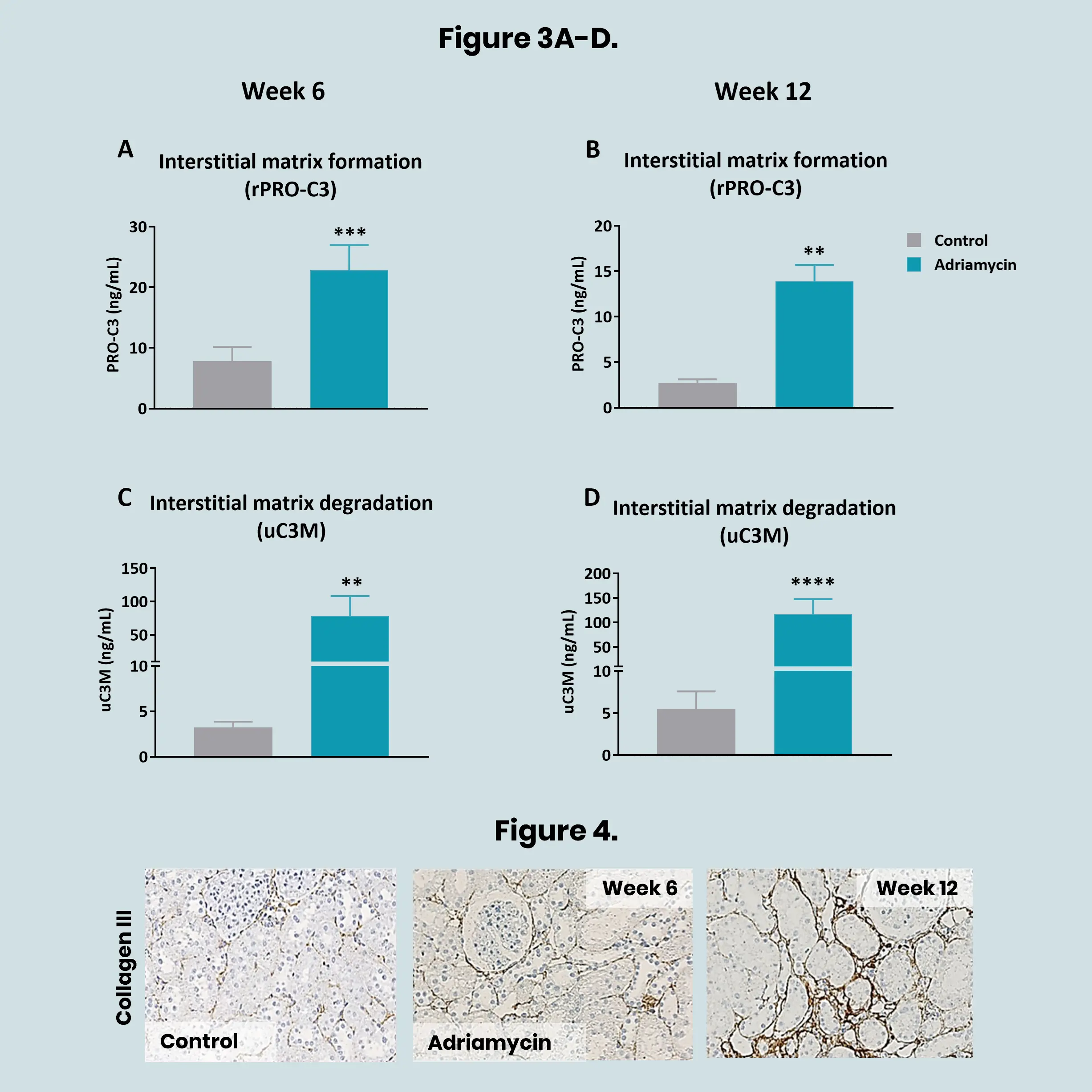
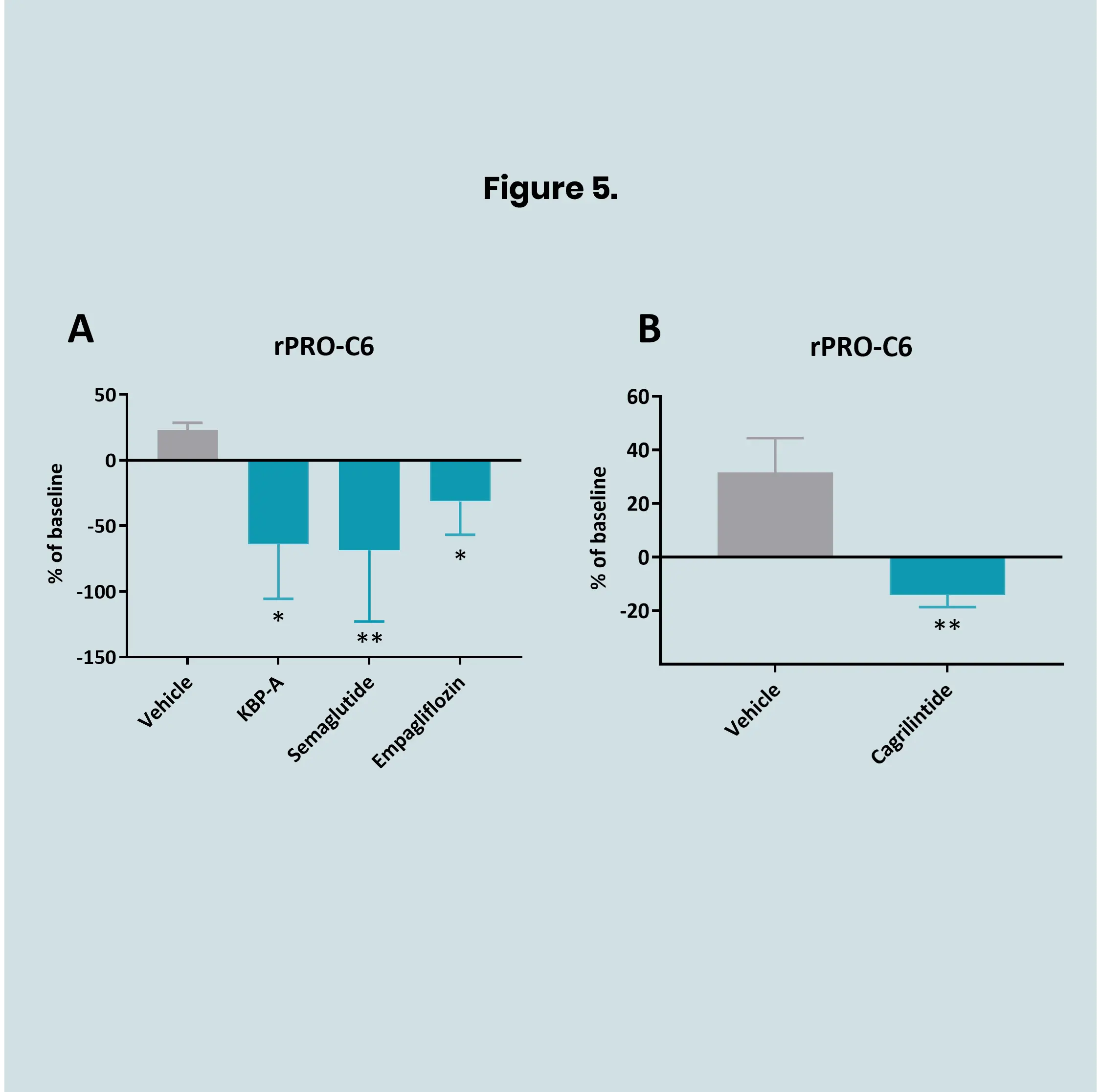
Preclinical translational models play a vital role in understanding kidney disease mechanisms and evaluating the efficacy of novel therapeutics before entering clinical trials. In models of kidney fibrosis, fibroblast activation and extracellular matrix (ECM) remodelling are key processes that closely reflect human disease progression. ECM-derived biomarkers offer a non-invasive and quantitative way to assess fibrogenesis and fibrolysis.
By integrating these biomarkers into preclinical studies, pharmaceutical developers and researchers can better monitor therapeutic effects, gain mechanistic insights, and improve the translational relevance of animal models. This integrated approach supports earlier and more confident go/no-go decisions, ultimately reducing development time and increasing the likelihood of clinical success.